Oxidative Stress-mediated Lipid Peroxidation-derived Lipid Aldehydes in the Pathophysiology of Neurodegenerative Diseases
- PMID: 39440770
- PMCID: PMC12163476
- DOI: 10.2174/011570159X342720241014164650
Oxidative Stress-mediated Lipid Peroxidation-derived Lipid Aldehydes in the Pathophysiology of Neurodegenerative Diseases
Abstract
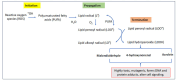
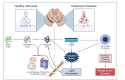
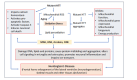
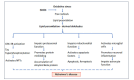
Neurodegenerative diseases such as Alzheimer's, Parkinson's, and amyotrophic lateral sclerosis cause damage and gradual loss of neurons affecting the central nervous system. Neurodegenerative diseases are most commonly seen in the ageing process. Ageing causes increased reactive oxygen species and decreased mitochondrial ATP generation, resulting in redox imbalance and oxidative stress. Oxidative stress-generated free radicals cause damage to membrane lipids containing polyunsaturated fatty acids, leading to the formation of toxic lipid aldehyde products such as 4- hydroxynonenal and malondialdehyde. Several studies have shown that lipid peroxidation-derived aldehyde products form adducts with cellular proteins, altering their structure and function. Thus, these lipid aldehydes could act as secondary signaling intermediates, modifying important metabolic pathways, and contributing to the pathophysiology of several human diseases, including neurodegenerative disorders. Additionally, they could serve as biomarkers for disease progression. This narrative review article discusses the biological and clinical significance of oxidative stress-mediated lipid peroxidation-derived lipid aldehydes in the pathophysiology of various neurodegenerative diseases.
Keywords: Alzheimer’s; Oxidative stress; Parkinson’s; hydroxynonenal; lipid peroxidation; malondialdehyde; neurodegenerative disorders..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures


Similar articles
-
4-hydroxynonenal and neurodegenerative diseases.Mol Aspects Med. 2003 Aug-Oct;24(4-5):293-303. doi: 10.1016/s0098-2997(03)00024-4. Mol Aspects Med. 2003. PMID: 12893007 Review.
-
Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal.J Neurochem. 1997 Jul;69(1):273-84. doi: 10.1046/j.1471-4159.1997.69010273.x. J Neurochem. 1997. PMID: 9202320
-
Amyloid beta peptide, 4-hydroxynonenal and apoptosis.Curr Alzheimer Res. 2006 Sep;3(4):359-64. doi: 10.2174/156720506778249506. Curr Alzheimer Res. 2006. PMID: 17017866 Review.
-
Lipid peroxidation and neurodegenerative disease.Free Radic Biol Med. 2011 Oct 1;51(7):1302-19. doi: 10.1016/j.freeradbiomed.2011.06.027. Epub 2011 Jun 30. Free Radic Biol Med. 2011. PMID: 21782935 Review.
-
4-Hydroxy-2-nonenal, a reactive product of lipid peroxidation, and neurodegenerative diseases: a toxic combination illuminated by redox proteomics studies.Antioxid Redox Signal. 2012 Dec 1;17(11):1590-609. doi: 10.1089/ars.2011.4406. Epub 2012 Feb 15. Antioxid Redox Signal. 2012. PMID: 22114878 Free PMC article. Review.
Cited by
-
Guardian of myelin and neural Integrity: foxo1a through slc7a11 mitigating oxidative damage in myelin.Redox Biol. 2025 Jul 12;85:103763. doi: 10.1016/j.redox.2025.103763. Online ahead of print. Redox Biol. 2025. PMID: 40669207 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

