Evaluation and Real-world Experience of a Neutralization Susceptibility Screening Assay for Broadly Neutralizing Anti-HIV-1 Antibodies
- PMID: 39441137
- PMCID: PMC11841631
- DOI: 10.1093/infdis/jiae486
Evaluation and Real-world Experience of a Neutralization Susceptibility Screening Assay for Broadly Neutralizing Anti-HIV-1 Antibodies
Abstract
Background: Development of a screening assay for the clinical use of broadly neutralizing antibodies (bnAbs) is a priority for HIV therapy and cure initiatives.
Methods: We assessed the PhenoSense Monoclonal Antibody Assay (Labcorp-Monogram Biosciences), which is Clinical Laboratory Improvement Amendments (CLIA) validated and has been used prospectively and retrospectively in multiple recent bnAb clinical trials.
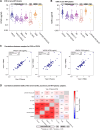
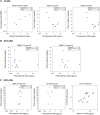
Results: When performed on plasma and longitudinal peripheral blood mononuclear cell samples (before and during antiretroviral therapy, respectively), as sourced from a recent clinical trial, the PhenoSense assay produced robust reproducibility, concordance across sample types, and expected ranges in the susceptibility measures of bnAbs in clinical development. When applied retrospectively to baseline samples from 3 recent studies, the PhenoSense assay correlated with published laboratory-based study evaluations, but baseline bnAb susceptibility was not consistently predictive of durable virus suppression. Assessment of assay feasibility in 4 recent clinical studies provides estimates of assay success rate and processing time.
Conclusions: The PhenoSense Monoclonal Antibody Assay provides reproducible bnAb susceptibility measurements across relevant sample types yet is not consistently predictive of virus suppression. Logistical and operational assay requirements can affect timely clinical trial conduct. These results inform bnAb studies in development.
Keywords: HIV; broadly neutralizing antibodies; clinical trials; screening assays.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. Y. L., J. D. R., and C. J. P. are employees of Monogram Biosciences and shareholders of LabCorp, its parent company. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI164565/AI/NIAID NIH HHS/United States
- National Institute of Allergy and Infectious Diseases
- UM1 AI068634/NH/NIH HHS/United States
- UM1 AI164570/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- R313-2019-790/Lundbeck Foundation
- R01 AI162646/AI/NIAID NIH HHS/United States
- UM1 AI069496/AI/NIAID NIH HHS/United States
- P30AI045008/Penn CFAR
- UM1 AI069419/AI/NIAID NIH HHS/United States
- P01 AI131338/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UM1AI164570/BEAT-HIV Delaney
- U01 AI169767/AI/NIAID NIH HHS/United States
- P30 AI124414/AI/NIAID NIH HHS/United States
- Statistical and Data Management Center
- UM1 AI106701/AI/NIAID NIH HHS/United States
- U01 AI169385/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

