Bispecific Antibodies as Bridging to BCMA CAR-T Cell Therapy for Relapsed/Refractory Multiple Myeloma
- PMID: 39441177
- PMCID: PMC11707513
- DOI: 10.1158/2643-3230.BCD-24-0118
Bispecific Antibodies as Bridging to BCMA CAR-T Cell Therapy for Relapsed/Refractory Multiple Myeloma
Abstract
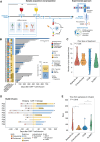
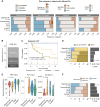
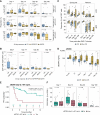
Establishing a strategy for sequencing of T cell-redirecting therapies for relapsed/refractory multiple myeloma (RRMM) is a pressing clinical need. We longitudinally tracked the clinical and immunologic impact of bispecific T cell-engaging antibodies (BsAb) as bridging therapy (BT) to subsequent B-cell maturation antigen-directed chimeric antigen receptor T (CAR-T) cell therapies in 52 patients with RRMM. BsAbs were a potent and safe option for BT, achieving the highest overall response rate (100%) to BT compared with chemotherapy, anti-CD38, or anti-SLAMF7 antibody-based regimens (46%). We observed early CD4+CAR+ and delayed CD8+CAR+ T-cell expansion in patients receiving BsAbs as BT. In vitro cytotoxicity of CAR-T cells was comparable among BT options. Single-cell analyses revealed increased clonality in the CD4+ and CD8+ T-cell compartments in patients with previous exposure to BsAbs at leukapheresis and on day 30 after CAR-T cell infusion. This study demonstrates the feasibility and efficacy of BT with BsAbs for CAR-T cell therapy in RRMM. Significance: CAR-T cell therapy and BsAbs have revolutionized treatment of triple-class refractory multiple myeloma; however, optimal sequencing is unknown. We demonstrate that BT with BsAb before B-cell maturation antigen-directed CAR-T cell therapy is safe and effective, which might have implications for other hematologic malignancies as well. See related commentary by Bal and Costa, p. 10.
©2024 American Association for Cancer Research.
Conflict of interest statement
M. Rade reports grants from European Union (CERTAINTY project, Grant Agreement 101136379) during the conduct of the study. M. Kreuz reports grants from European Union (CERTAINTY project, Grant Agreement 101136379) during the conduct of the study. K.H. Metzeler reports grants and personal fees from AbbVie and personal fees from Bristol Myers Squibb, Astellas, Janssen, Novartis, Otsuka, Pfizer, Menarini StemLine, and Servier outside the submitted work. G.-N. Franke reports personal fees from Novartis Pharma GmbH, Germany, Incyte Biosciences GmbH, Germany, and Pfizer, Germany outside the submitted work. U. Köhl reports grants from EU grant imSAVAR, EU grant CERTAINTY, EU grant T2Evolve, BMBF grant SaxoCell, DAAD grant project 57616814, and German José-Carreras Leukemia Foundation during the conduct of the study, as well as grants from AstraZeneca GmbH, Affimed GmbH, ATMPS Ltd., Glycostem Therapeutics, Miltenyi Biotec GmbH, Novartis AG, Zelluna Immunotherapy, and Novo Nordisk Foundation Cellerator outside the submitted work. K. Reiche reports grants from European Union (CERTAINTY project, Grant Agreement 101136379) during the conduct of the study. U. Platzbecker reports grants and personal fees from Bristol Myers Squibb, Janssen, and Curis during the conduct of the study. V. Vucinic reports personal fees from Janssen Cillag, Bristol Myers Squibb, Celgene, Gilead Kite, and Novartis during the conduct of the study, as well as personal fees from MSD, Sobi, Elli Lilly and Company, Mallinckrodt, AstraZeneca, AbbVie, and BeiGene outside the submitted work. M. Merz reports grants from Janssen, SpringWorks, and Roche during the conduct of the study, as well as personal fees from Janssen, Bristol Myers Squibb, Amgen, Takeda, and Pfizer outside the submitted work. No disclosures were reported by the other authors.
Figures



References
-
- Chari A, Minnema MC, Berdeja JG, Oriol A, van de Donk NWCJ, Rodríguez-Otero P, et al. Talquetamab, a T-cell-redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med 2022;387:2232–44. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

