Normalized Interferon Signatures and Clinical Improvements by IFNAR1 Blocking Antibody (Anifrolumab) in Patients with Type I Interferonopathies
- PMID: 39441221
- PMCID: PMC11499543
- DOI: 10.1007/s10875-024-01826-2
Normalized Interferon Signatures and Clinical Improvements by IFNAR1 Blocking Antibody (Anifrolumab) in Patients with Type I Interferonopathies
Abstract
Purpose: A causal role of type-I interferons (IFN-I) in autoinflammatory type-I interferonopathies such as SAVI (STING-associated vasculopathy with onset in infancy) and CANDLE (chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperatures) is suggested by elevated expression of IFN-I stimulated genes (ISGs). Hitherto, the lack of specific inhibitors of IFN-I signaling has prevented the verification of a causal role for IFN-I in these conditions. Commonly used inhibitors of the JAK/STAT pathway exert broad effects on multiple signaling pathways leading to more general immunosuppression beyond IFN-I signaling.
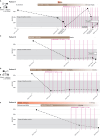
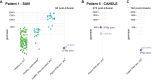
Methods: Here we show in four patients with SAVI and one patient with CANDLE syndrome that blockade of the IFNAR1 receptor (Anifrolumab) exerts an additive effect over JAK-inhibitor alone. In two patients with SAVI, monotherapy with Anifrolumab is sufficient to retain a suppressed IFN-I signature and clinical improvement.
Results: Anifrolumab normalizes IFN-I signature genes and relieves symptoms beyond what is typically achieved by a JAK-inhibitor (Baricitinib) alone in patients with type-I interferonopathies. In two patients Anifrolumab was used successfully as monotherapy. Addition of Anifrolumab enabled steroid tapering and cessation with reduced overall immunosuppression and lower risks of opportunistic infections and improved metabolic states and growth which is highly beneficial in these young patients.
Conclusion: These results verify a causal role of IFN-I signaling in type-I Interferonopathies SAVI and CANDLE and suggests Anifrolumab as an important new treatment option in autoinflammatory diseases with elevated IFN-I induced gene expression. Genia Kretzschmar, Laura Piñero Páez, and Ziyang Tan are shared-first authors. Sara Alehashemi, AnnaCarin Horne, and Petter Brodin are co-senior author.
Keywords: IFN-I, type I IFN, IFNAR1, Interferonopathies, Anifrolumab.
© 2024. The Author(s).
Conflict of interest statement
P.B, T.L and J.M are cofounders of Cytodelics AB (Stockholm, Sweden) which produce and distribute whole blood cell stabilizer solutions used to preserve samples for cytometry analyses within this study.
Figures

References
-
- Crow YJ. Type I interferonopathies: a novel set of inborn errors of immunity. Ann N York Acad Sci. 2011;1238(1):91–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

