Autoantibody against aquaporin-5 may be a new diagnostic biomarker for primary Sjögren's syndrome
- PMID: 39441465
- PMCID: PMC11582186
- DOI: 10.1007/s10067-024-07190-1
Autoantibody against aquaporin-5 may be a new diagnostic biomarker for primary Sjögren's syndrome
Abstract
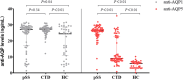
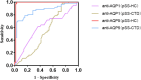
The study aims to assess the diagnostic and clinical significance of autoantibodies against aquaporin-1 (anti-AQP1) and aquaporin-5 (anti-AQP5) in primary Sjögren's syndrome (pSS). A total of 163 participants were categorized into three groups: pSS group, other connective tissue diseases (CTD) group, and healthy control (HC) group. The levels of anti-AQP1 and anti-AQP5 autoantibodies in serum were determined using enzyme-linked immunosorbent assay (ELISA), and clinical data from patients were collected for statistical analysis. Our results showed that the level of anti-AQP1 in the pSS group was higher than in the HC group (P < 0.05), and no significant difference was observed between the pSS group and the CTD group (P > 0.05). ROC showed that the anti-AQP1 had no diagnostic value for pSS (P > 0.05). The anti-AQP5 level of 39 healthy adults was all below the cut-off value (14.10 ng/ml) (P < 0.05). The level of anti-AQP5 in the pSS group was higher than the CTD group (P < 0.05), the AUC was 0.86 (95% CI 0.80-0.93), with a sensitivity of 0.95 (95% CI 0.87-0.99) and a specificity of 0.70 (95% CI 0.58-0.84). No correlation was found between anti-AQP5 levels and the EULAR primary Sjögren's syndrome disease activity index score, anti-SSA, anti-SSB, antinuclear antibodies, rheumatoid factor, anti-ds-DNA, salivary gland flow rate, complement 3, and lymphocyte count in pSS samples (P > 0.05), respectively. Therefore, the elevated anti-AQP5 may emerge as a novel diagnostic biomarker for pSS patients due to high sensitivity and specificity.
Keywords: Anti-aquaporin autoantibody; Biomarker; Primary Sjögren’s syndrome.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was granted approval by the Ethics Committee of the First Affiliated Hospital of Army Medical University (No: (A) KY2021075). Informed consent was obtained from all the patients before conducting the study. Disclosures: None.
Figures
References
-
- Manfrè V, Chatzis LG, Cafaro G, Fonzetti S, Calvacchi S, Fulvio G et al (2022) Sjögren’s syndrome: one year in review 2022. Clin Exp Rheumatol 40(12):2211–2224. 10.55563/clinexprheumatol/43z8gu - PubMed
-
- Zhang W, Chen Z, Li XM, Gao J, Zhao Y (2023) Recommendations for the diagnosis and treatment of Sjögren’s syndrome in China. Zhonghua Nei Ke Za Zhi 62(9):1059–1067 - PubMed
-
- Brito-Zerón P, Acar-Denizli N, Ng WF, Zeher M, Rasmussen A, Mandl T, et al (2018) How immunological profile drives clinical phenotype of primary Sjögren’s syndrome at diagnosis: analysis of 10,500 patients (Sjögren Big Data Project). Clin Exp Rheumatol.36 Suppl 112(3):102–112. - PubMed
-
- Cornec D, Jousse-Joulin S, Pers JO, Marhadour T, Cochener B, Boisramé-Gastrin S et al (2013) Contribution of salivary gland ultrasonography to the diagnosis of Sjögren’s syndrome: toward new diagnostic criteria? Arthritis Rheum 65(1):216–225. 10.1002/art.37698 - PubMed
-
- He J, Jiang J, Baumgart K (2022) Candidate autoantibodies for primary Sjögren’s syndrome: where are they now? Clin Exp Rheumatol 40(12):2387–2394. 10.55563/clinexprheumatol/vmqtz4 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

