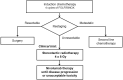
Stereotactic Radiotherapy Plus Nivolumab in Patients with Locally Advanced Pancreatic Cancer: Results from Phase 1/2 Clinical CA209-9KH Trial
- PMID: 39441483
- PMCID: PMC11574225
- DOI: 10.1007/s40487-024-00309-z
Stereotactic Radiotherapy Plus Nivolumab in Patients with Locally Advanced Pancreatic Cancer: Results from Phase 1/2 Clinical CA209-9KH Trial
Abstract
Introduction: The dismal prognosis of pancreatic ductal adenocarcinoma (PDAC) highlights the urgent need for novel therapeutic strategies. Immune checkpoint inhibitors (ICIs) seem to be ineffective in most PDAC studies. Therefore, we conducted an open-label, multicenter phase 1/2 study (CA209-9KH) to evaluate the safety of stereotactic radiotherapy (SRT) and sequential ICI therapy in PDAC, as well as to validate the efficacy of this regimen as a potential activator of antitumor immunity.
Methods: Patients aged ≥ 18 years with unresectable non-metastatic PDAC following four FOLFIRINOX induction cycles were included. Treatment comprised SRT (4 × 8 Gy) and sequential nivolumab administration until disease progression or unacceptable toxicity. The primary endpoints were safety and toxicity assessment. Secondary endpoints included progression-free survival (PFS), overall survival (OS), biomarker evaluation, and quality of life (QoL) analysis.
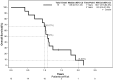
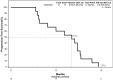
Results: Twenty-two patients were screened, with 15 enrolled. Eleven (median) nivolumab cycles were administered. SRT demonstrated low and clinically nonsignificant toxicity, whereas nivolumab toxicity aligned with prior safety profiles, without grade 4-5 events observed. Three patients discontinued therapy owing to toxicity. Median PFS and OS were 8.1 and 13.0 months, respectively, with 12-month PFS and OS rates of 0% and 66.7%, respectively, and a 24-month OS rate of 8.9%. Biomarker levels correlated with clinical or radiological disease control. Patient-reported QoL remained acceptable, deteriorating with disease progression.
Conclusion: SRT and nivolumab therapy exhibited manageable toxicity profiles consistent with previous findings; however, long-term treatment responses were not achieved with this regimen in locally advanced PDAC. Another strategy to trigger antitumor immunity in PDAC needs to be sought.
Trial registration: EudraCT: 2017-003404-52; ClinicalTrials.gov: NCT04098432.
Keywords: Nivolumab; Pancreatic ductal adenocarcinoma; Stereotactic radiotherapy.
© 2024. The Author(s).
Conflict of interest statement
Figures
References
-
- Global Cancer Observatory. Available at: https://www-dep.iarc.fr/. Accessed November 21, 2023.
-
- Huang J, Lok V, Ngai CH, Zhang L, Yuan J, Lao XQ, et al. Worldwide burden of, risk factors for, and trends in pancreatic cancer. Gastroenterology. 2021;160:744–54. 10.1053/j.gastro.2020.10.007. - PubMed
-
- Dušek L, Mužík J, Kubásek M, Koptíková J, Žaloudík J, Vyzula R. Epidemiologie zhoubných nádorů v České republice [online]. Masaryk University. Available at: http://www.svod.cz. Accessed April 13, 2024.
-
- Yasinzai AQK, Tareen B, Tracy K, Jamil N, Khan M, Ullah H, et al. Pancreatic ductal adenocarcinoma: exploring clinicopathological trends and racial disparities in a comprehensive US population-based study. Clin Transl Oncol. 2024. 10.1007/s12094-024-03484-7. (Online ahead of print). - PubMed
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. 10.3322/caac.21708. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical