Pregabalin for Neuropathic Pain and Itch in Recessive Dystrophic Epidermolysis Bullosa: A Randomized Crossover Trial
- PMID: 39441591
- PMCID: PMC11581501
- DOI: 10.1001/jamadermatol.2024.3767
Pregabalin for Neuropathic Pain and Itch in Recessive Dystrophic Epidermolysis Bullosa: A Randomized Crossover Trial
Abstract
Importance: Patients with recessive dystrophic epidermolysis bullosa (RDEB) experience neuropathic pain and itch. There is a lack of evidence on any treatment for these symptoms in patients with RDEB.
Objectives: To test the efficacy of pregabalin in the treatment of neuropathic pain and itch in patients with RDEB.
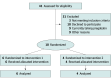
Design, setting, and participants: A randomized, double-blinded, crossover trial of oral pregabalin (50-300 mg/d) vs placebo was conducted at 2 sites, Toronto (Canada) and Santiago (Chile) from January 1, 2019, to December 31, 2020. Patients eligible to participate were diagnosed with RDEB, aged 8 to 40 years, not pregnant or lactating (if female), and had evidence of probable neuropathic pain and itching defined as distal thermal sensory loss (confirmed by thermal roller), score of 4 or greater on the Douleur Neuropathique 4 questionnaire (DN4), and score greater than 4 on the 10-point visual analog scale [VAS]). Patients with a clinically important or poorly controlled medical or psychiatric condition or pregabalin intolerance or allergy were excluded. Of 41 patients screened, 3 were not eligible and 28 declined enrollment. Data analyses were performed in 2021 through 2023.
Intervention: Participants received both pregabalin and matched placebo (titrated to a maximum-tolerated dose of 300 mg/day) in a randomized sequence so that comparisons could be made within participants and between groups.
Main outcomes and measures: Difference in the mean pain and itch scores between pregabalin and placebo treatment (measured using VAS) before and after intervention.
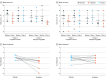
Results: In all, 10 participants were randomized to 2 groups, 6 patients (mean [SD] age, 26.7 [8.1] years; 3 females [50%]) in group 1, and 4 patients (mean [SD] age, 26.5 [7.8] years, 2 females [50%]) in group 2. Group 1 received a sequence of pregabalin-placebo while group 2 received placebo-pregabalin. Pregabalin significantly reduced mean (SD) pain scores by 1.9 (1.5) points when controlling for sequence and treatment period vs baseline, while placebo had 0.1 (2.0) points of reduction. The effect of pregabalin was a mild but significant reduction in itch compared to baseline (mean [SD] points, 0.9 [2.2]), whereas the placebo produced no reduction (0.1 [2.5]). The mean pregabalin dose was generally well tolerated.
Conclusions and relevance: The results of this randomized crossover trial indicate that pregabalin significantly reduced pain and itch scores from baseline compared to placebo in patients with RDEB. This feasibility study provided preliminary data on the efficacy of pregabalin in managing pain and itch in RDEB and gathered essential data to inform the design of a larger cohort trial.
Trial registration: ClinicalTrials.gov Identifier: NCT03928093.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

