FlaG competes with FliS-flagellin complexes for access to FlhA in the flagellar T3SS to control Campylobacter jejuni filament length
- PMID: 39441631
- PMCID: PMC11536152
- DOI: 10.1073/pnas.2414393121
FlaG competes with FliS-flagellin complexes for access to FlhA in the flagellar T3SS to control Campylobacter jejuni filament length
Abstract
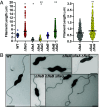
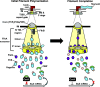
Bacteria power rotation of an extracellular flagellar filament for swimming motility. Thousands of flagellin subunits compose the flagellar filament, which extends several microns from the bacterial surface. It is unclear whether bacteria actively control filament length. Many polarly flagellated bacteria produce shorter flagellar filaments than peritrichous bacteria, and FlaG has been reported to limit flagellar filament length in polar flagellates. However, a mechanism for how FlaG may function is unknown. We observed that deletion of flaG in the polarly flagellated pathogens Vibrio cholerae, Pseudomonas aeruginosa, and Campylobacter jejuni caused extension of flagellar filaments to lengths comparable to peritrichous bacteria. Using C. jejuni as a model to understand how FlaG controls flagellar filament length, we found that FlaG and FliS chaperone-flagellin complexes antagonize each other for interactions with FlhA in the flagellar type III secretion system (fT3SS) export gate. FlaG interacted with an understudied region of FlhA, and this interaction appeared to be enhanced in ΔfliS and FlhA FliS-binding mutants. Our data support that FlaG evolved in polarly flagellated bacteria as an antagonist to interfere with the ability of FliS to interact with and deliver flagellins to FlhA in the fT3SS export gate to control flagellar filament length so that these bacteria produce relatively shorter flagella than peritrichous counterparts. This mechanism is similar to how some gatekeepers in injectisome T3SSs prevent chaperones from delivering effector proteins until completion of the T3SS and host contact occurs. Thus, flagellar and injectisome T3SSs have convergently evolved protein antagonists to negatively impact respective T3SSs to secrete their major terminal substrates.
Keywords: Campylobacter jejuni; FlaG; FlhA; FliS; flagellar filament length.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

