Curvature fluctuations of fluid vesicles reveal hydrodynamic dissipation within the bilayer
- PMID: 39441635
- PMCID: PMC11536141
- DOI: 10.1073/pnas.2413557121
Curvature fluctuations of fluid vesicles reveal hydrodynamic dissipation within the bilayer
Abstract
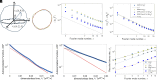
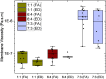
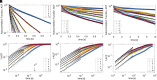
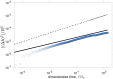
The biological function of membranes is closely related to their softness, which is often studied through the membranes' thermally driven fluctuations. Typically, the analysis assumes that the relaxation rate of a pure bending deformation is determined by the competition between membrane bending rigidity and viscous dissipation in the surrounding medium. Here, we reexamine this assumption and demonstrate that viscous flows within the membrane dominate the dynamics of bending fluctuations of nonplanar membranes with a radius of curvature smaller than the Saffman-Delbrück length. Using flickering spectroscopy of giant vesicles made of dipalmitoylphosphatidylcholine, DPPC:cholesterol mixtures and pure diblock-copolymer membranes, we experimentally detect the signature of membrane dissipation in curvature fluctuations. We show that membrane viscosity can be reliably obtained from the short time behavior of the shape time correlations. The results indicate that the DPPC:cholesterol membranes behave as a Newtonian fluid, while the polymer membranes exhibit more complex rheology. Our study provides physical insights into the time scales of curvature remodeling of biological and synthetic membranes.
Keywords: curvature fluctuations; lipid bilayer; membrane viscosity; polymersome; vesicle.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
References
-
- Lipowsky R., The conformation of membranes. Nature 349, 475–481 (1991). - PubMed
-
- Rideau E., Dimova R., Schwille P., Wurm F. R., Landfester K., Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 47, 8572–8610 (2018). - PubMed
-
- Bonifacino J. S., Glick B. S., The mechanisms of vesicle budding and fusion. Cell 116, 153–166 (2004). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

