Daily glycome and transcriptome profiling reveals polysaccharide structures and correlated glycosyltransferases critical for cotton fiber growth
- PMID: 39441672
- PMCID: PMC11629744
- DOI: 10.1111/tpj.17084
Daily glycome and transcriptome profiling reveals polysaccharide structures and correlated glycosyltransferases critical for cotton fiber growth
Abstract
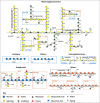
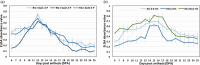
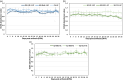
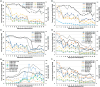
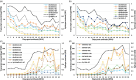
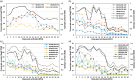
Cotton fiber is the most valuable naturally available material for the textile industry and the fiber length and strength are key determinants of its quality. Dynamic changes in the pectin, xyloglucan, xylan, and cellulose polysaccharide epitope content during fiber growth contribute to complex remodeling of fiber cell wall (CW) and quality. Detailed knowledge about polysaccharide compositional and structural alteration in the fiber during fiber elongation and strengthening is important to understand the molecular dynamics of fiber development and improve its quality. Here, large-scale glycome profiling coupled with fiber phenotype and transcriptome profiling was conducted on fiber collected daily covering the most critical window of fiber development. The profiling studies with high temporal resolution allowed us to identify specific polysaccharide epitopes associated with distinct fiber phenotypes that might contribute to fiber quality. This study revealed the critical role of highly branched RG-I pectin epitopes such as β-1,4-linked-galactans, β-1,6-linked-galactans, and arabinogalactans, in addition to earlier reported homogalacturonans and xyloglucans in the formation of cotton fiber middle lamella and contributing to fiber plasticity and elongation. We also propose the essential role of heteroxylans (Xyl-MeGlcA and Xyl-3Ar), as a guiding factor for secondary CW cellulose microfibril arrangement, thus contributing to fiber strength. Correlation analysis of profiles of polysaccharide epitopes from glycome data and expression profiles of glycosyltransferase-encoding genes from transcriptome data identified several key putative glycosyltransferases that are potentially involved in synthesizing the critical polysaccharide epitopes. The findings of this study provide a foundation to identify molecular factors that dictate important fiber traits.
Keywords: Gossypium hirsutum; cellulose; cotton fiber; glycome profiling; glycosyltransferases; pectin; polysaccharide epitopes; transcriptome profiling; xylan; xyloglucan.
© 2024 The Author(s). The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Abidi, N. , Cabrales, L. & Haigler, C.H. (2014) Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydrate Polymers, 100, 9–16. - PubMed
-
- An, C. , Saha, S. , Jenkins, J.N. , Scheffler, B.E. , Wilkins, T.A. & Stelly, D.M. (2007) Transcriptome profiling, sequence characterization, and SNP‐based chromosomal assignment of the EXPANSIN genes in cotton. Molecular Genetics and Genomics, 278, 539–553. - PubMed
-
- Applequist, W.L. , Cronn, R. & Wendel, J.F. (2001) Comparative development of fiber in wild and cultivated cotton. Evolution and Development, 3, 3–17. - PubMed
-
- Atalla, R.H. , Hackney, J.M. , Uhlin, I. & Thompson, N.S. (1993) Hemicelluloses as structure regulators in the aggregation of native cellulose. International Journal of Biological Macromolecules, 15, 109–112. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

