Actin dynamics switches two distinct modes of endosomal fusion in yolk sac visceral endoderm cells
- PMID: 39441732
- PMCID: PMC11498936
- DOI: 10.7554/eLife.95999
Actin dynamics switches two distinct modes of endosomal fusion in yolk sac visceral endoderm cells
Abstract
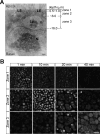
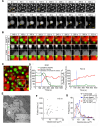
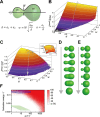
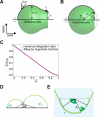
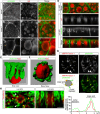
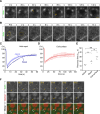
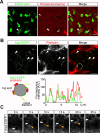
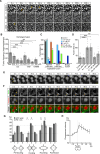
Membranes undergo various patterns of deformation during vesicle fusion, but how this membrane deformation is regulated and contributes to fusion remains unknown. In this study, we developed a new method of observing the fusion of individual late endosomes and lysosomes by using mouse yolk sac visceral endoderm cells that have huge endocytic vesicles. We found that there were two distinct fusion modes that were differently regulated. In homotypic fusion, two late endosomes fused quickly, whereas in heterotypic fusion they fused to lysosomes slowly. Mathematical modeling showed that vesicle size is a critical determinant of these fusion types and that membrane fluctuation forces can overcome the vesicle size effects. We found that actin filaments were bound to late endosomes and forces derived from dynamic actin remodeling were necessary for quick fusion during homotypic fusion. Furthermore, cofilin played a role in endocytic fusion by regulating actin turnover. These data suggest that actin promotes vesicle fusion for efficient membrane trafficking in visceral endoderm cells.
Keywords: actin; cell biology; fusion; late endosome; lysosome; mouse; mouse embryo; yolk sac visceral endoderm.
© 2024, Koike et al.
Conflict of interest statement
SK, MT, MT, TO, TN, KK, MM No competing interests declared
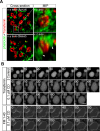
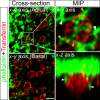
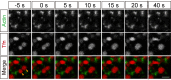
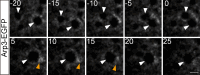
Figures


Update of
- doi: 10.1101/2023.12.17.572077
- doi: 10.7554/eLife.95999.1
- doi: 10.7554/eLife.95999.2
References
-
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, Yahara I. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nature Neuroscience. 2001;4:367–373. doi: 10.1038/86011. - DOI - PubMed
-
- Bielinska M, Narita N, Wilson DB. Distinct roles for visceral endoderm during embryonic mouse development. The International Journal of Developmental Biology. 1999;43:183–205. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

