Detection of circulating tumor cells in non-metastatic prostate cancer through integration of a microfluidic CTC enrichment system and multiparametric flow cytometry
- PMID: 39441869
- PMCID: PMC11498670
- DOI: 10.1371/journal.pone.0312296
Detection of circulating tumor cells in non-metastatic prostate cancer through integration of a microfluidic CTC enrichment system and multiparametric flow cytometry
Abstract
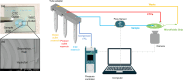
Prostate cancer (PCa) is the second most common cancer among men and the fifth leading cause of cancer death. Circulating tumor cell (CTC) enumeration and characterisation in PCa has been shown to provide valuable information on prognosis of disease, therapy management and detection of resistance. Here, Cellsway's microfluidic platform for high-throughput enrichment of intact CTC populations was used to isolate CTCs from the blood of 20 localised PCa patients and 10 healthy donor samples to evaluate the clinical performance of the technology. To enumerate and characterise CTCs, a multi-parameter flow cytometry analysis was performed on the enriched CTC suspension using CTC-specific biomarkers. CTCs were detected in 17 of 20 patient samples, which corresponds to 85% CTC positivity. The median CTC count per 7.5 ml blood was 2 (1-9). In 80% of patients (n = 16), the number of CTCs ranged from 1 to 5, and in 5% of patients (n = 1) the number of CTCs was above 5. No CTCs were observed in the blood samples of 10 healthy volunteers, demonstrating the high specificity and low risk of false positives of the technology.
Copyright: © 2024 Kilercik et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have read the journal’s policy and the authors of this manuscript have the following competing interests: EÖ, ŞŞ, NE, EM, BŞD, ÖZ and HK are employees of Mikro Biyosistemler A.S. MKilercik, MKolay and CC are employees of Acibadem Labmed Clinical Laboratories. The authors would like to declare the following patents/patent applications associated with this research: The microfluidic CTC enrichment chip used in this study is related to the patent (US 12,036,553 B2) and is under development as a commercial product by Mikro Biyosistemler A.S. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2021;79: 243–262. doi: 10.1016/j.eururo.2020.09.042 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical