Isolation and tracing of matrix-producing notochordal and chondrocyte cells using ACAN-2A-mScarlet reporter human iPSC lines
- PMID: 39441923
- PMCID: PMC11498221
- DOI: 10.1126/sciadv.adp3170
Isolation and tracing of matrix-producing notochordal and chondrocyte cells using ACAN-2A-mScarlet reporter human iPSC lines
Abstract
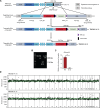
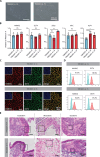
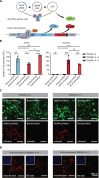
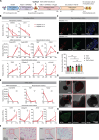
The development of human induced pluripotent stem cell (iPSC)-based regenerative therapies is challenged by the lack of specific cell markers to isolate differentiated cell types and improve differentiation protocols. This issue is particularly critical for notochordal-like cells and chondrocytes, which are crucial in treating back pain and osteoarthritis, respectively. Both cell types produce abundant proteoglycan aggrecan (ACAN), crucial for the extracellular matrix. We generated two human iPSC lines containing an ACAN-2A-mScarlet reporter. The reporter cell lines were validated using CRISPR-mediated transactivation and functionally validated during notochord and cartilage differentiation. The ability to isolate differentiated cell populations producing ACAN enables their enrichment even in the absence of specific cell markers and allows for comprehensive studies and protocol refinement. ACAN's prevalence in various tissues (e.g., cardiac and cerebral) underscores the reporter's versatility as a valuable tool for tracking matrix protein production in diverse cell types, benefiting developmental biology, matrix pathophysiology, and regenerative medicine.
Figures


References
-
- Luoma K., Riihimäki H., Luukkonen R., Raininko R., Viikari-Juntura E., Lamminen A., Low back pain in relation to lumbar disc degeneration. Spine 25, 487–492 (2000). - PubMed
-
- Le Maitre C., Pockert A., Buttle D., Freemont A., Hoyland J., Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem. Soc. Trans. 35, 652–655 (2007). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

