Compromised macrophages contribute to progression of MASH to hepatocellular carcinoma in FGF21KO mice
- PMID: 39441934
- PMCID: PMC11498219
- DOI: 10.1126/sciadv.ado9311
Compromised macrophages contribute to progression of MASH to hepatocellular carcinoma in FGF21KO mice
Abstract
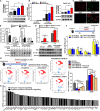
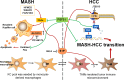
Metabolic dysfunction-associated steatohepatitis is well accepted as a potential precursor of hepatocellular carcinoma. Previously, we reported that fibroblast growth factor 21 (FGF21) revealed a novel anti-inflammatory activity via inhibiting the TLR4-IL-17A signaling, which could be a potential anticarcinogenetic mechanism to prevent to MASH-HCC transition. Here, we set out to determine whether FGF21 has a major impact on Kupffer cells' (KCs) ability during MASH-HCC transition. We found aberrant hepatic FGF21 and KC pool in human MASH-HCC. Lack of FGF21 up-regulated ALOX15, which converted the oxidized fatty acids to induce excessive KC death and mobilization of monocyte-derived macrophages (MoMFs) for KC replacement. Lack of FGF21 oversupplied free fatty acids for sphingosine-1-phosphate (S1P) cascade synthesis to mediate MASH-HCC transition via S1P-YAP signaling and cross-talk between tumor cells and macrophages. In conclusion, lack of FGF21 accelerated MASH-HCC transition via the S1P-YAP signaling. Compromised MoMFs could present as tumor-associated macrophage phenotype rendering tumor immune microenvironment for MASH-HCC transition.
Figures





References
-
- Marengo A., Rosso C., Bugianesi E., Liver cancer: Connections with obesity, fatty liver, and cirrhosis. Annu. Rev. Med. 67, 103–117 (2016). - PubMed
-
- Hirode G., Vittinghoff E., Wong R. J., Increasing clinical and economic burden of nonalcoholic fatty liver disease among hospitalized adults in the United States. J. Clin. Gastroenterol. 53, 765–771 (2019). - PubMed
-
- Ludwig J., Viggiano T. R., McGill D. B., Oh B. J., Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 55, 434–438 (1980). - PubMed
-
- Tilg H., Moschen A., Update on nonalcoholic fatty liver disease: Genes involved in nonalcoholic fatty liver disease and associated inflammation. Curr. Opin. Clin. Nutr. Metab. Care 13, 391–396 (2010). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

