Acute contact with profibrotic macrophages mechanically activates fibroblasts via αvβ3 integrin-mediated engagement of Piezo1
- PMID: 39441936
- PMCID: PMC11498225
- DOI: 10.1126/sciadv.adp4726
Acute contact with profibrotic macrophages mechanically activates fibroblasts via αvβ3 integrin-mediated engagement of Piezo1
Abstract
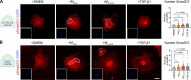
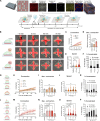
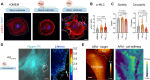
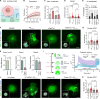
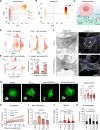
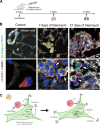
Fibrosis-excessive scarring after injury-causes >40% of disease-related deaths worldwide. In this misguided repair process, activated fibroblasts drive the destruction of organ architecture by accumulating and contracting extracellular matrix. The resulting stiff scar tissue, in turn, enhances fibroblast contraction-bearing the question of how this positive feedback loop begins. We show that direct contact with profibrotic but not proinflammatory macrophages triggers acute fibroblast contractions. The contractile response depends on αvβ3 integrin expression on macrophages and Piezo1 expression on fibroblasts. The touch of macrophages elevates fibroblast cytosolic calcium within seconds, followed by translocation of the transcription cofactors nuclear factor of activated T cells 1 and Yes-associated protein, which drive fibroblast activation within hours. Intriguingly, macrophages induce mechanical stress in fibroblasts on soft matrix that alone suppresses their spontaneous activation. We propose that acute contact with suitable macrophages mechanically kick-starts fibroblast activation in an otherwise nonpermissive soft environment. The molecular components mediating macrophage-fibroblast mechanotransduction are potential targets for antifibrosis strategies.
Figures




References
-
- Moss B. J., Ryter S. W., Rosas I. O., Pathogenic mechanisms underlying idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 17, 515–546 (2022). - PubMed
-
- Younesi F. S., Miller A. E., Barker T. H., Rossi F. M. V., Hinz B., Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat. Rev. Mol. Cell Biol. 25, 617–638 (2024). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

