An autonomous implantable device for the prevention of death from opioid overdose
- PMID: 39441938
- PMCID: PMC11498215
- DOI: 10.1126/sciadv.adr3567
An autonomous implantable device for the prevention of death from opioid overdose
Abstract
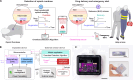
Opioid overdose accounts for nearly 75,000 deaths per year in the United States, now a leading cause of mortality among young people aged 18 to 45 years. At overdose levels, opioid-induced respiratory depression becomes fatal without the administration of naloxone within minutes. Currently, overdose survival relies on bystander intervention, requiring a nearby person to find the overdosed individual and have immediate access to naloxone to administer. To circumvent the bystander requirement, we developed the Naloximeter: a class of life-saving implantable devices that autonomously detect and treat overdose while simultaneously contacting first responders. We present three Naloximeter platforms, for fundamental research and clinical translation, all equipped with optical sensors, drug delivery mechanisms, and a supporting ecosystem of technology to counteract opioid-induced respiratory depression. In small and large animal studies, the Naloximeter rescues from otherwise fatal opioid overdose within minutes. This work introduces life-changing, clinically translatable technologies that can broadly benefit a susceptible population recovering from opioid use disorder.
Figures



Update of
-
An Autonomous Implantable Device for the Prevention of Death from Opioid Overdose.bioRxiv [Preprint]. 2024 Jul 2:2024.06.27.600919. doi: 10.1101/2024.06.27.600919. bioRxiv. 2024. Update in: Sci Adv. 2024 Oct 25;10(43):eadr3567. doi: 10.1126/sciadv.adr3567. PMID: 39005313 Free PMC article. Updated. Preprint.
References
-
- Strang J., Volkow N. D., Degenhardt L., Hickman M., Johnson K., Koob G. F., Marshall B. D. L., Tyndall M., Walsh S. L., Opioid use disorder. Nat. Rev. Dis. Primer 6, 1–28 (2020). - PubMed
-
- France C. P., Ahern G. P., Averick S., Disney A., Enright H. A., Esmaeli-Azad B., Federico A., Gerak L. R., Husbands S. M., Kolber B., Lau E. Y., Lao V., Maguire D. R., Malfatti M. A., Martinez G., Mayer B. P., Pravetoni M., Sahibzada N., Skolnick P., Snyder E. Y., Tomycz N., Valdez C. A., Zapf J., Countermeasures for preventing and treating opioid overdose. Clin. Pharmacol. Ther. 109, 578–590 (2021). - PMC - PubMed
-
- Blanco C., Volkow N. D., Management of opioid use disorder in the USA: Present status and future directions. Lancet 393, 1760–1772 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

