Effects of microgravity on human iPSC-derived neural organoids on the International Space Station
- PMID: 39441987
- PMCID: PMC11631337
- DOI: 10.1093/stcltm/szae070
Effects of microgravity on human iPSC-derived neural organoids on the International Space Station
Abstract
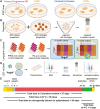
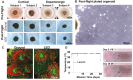
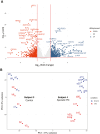
Research conducted on the International Space Station (ISS) in low-Earth orbit (LEO) has shown the effects of microgravity on multiple organs. To investigate the effects of microgravity on the central nervous system, we developed a unique organoid strategy for modeling specific regions of the brain that are affected by neurodegenerative diseases. We generated 3-dimensional human neural organoids from induced pluripotent stem cells (iPSCs) derived from individuals affected by primary progressive multiple sclerosis (PPMS) or Parkinson's disease (PD) and non-symptomatic controls, by differentiating them toward cortical and dopaminergic fates, respectively, and combined them with isogenic microglia. The organoids were cultured for a month using a novel sealed cryovial culture method on the International Space Station (ISS) and a parallel set that remained on Earth. Live samples were returned to Earth for analysis by RNA expression and histology and were attached to culture dishes to enable neurite outgrowth. Our results show that both cortical and dopaminergic organoids cultured in LEO had lower levels of genes associated with cell proliferation and higher levels of maturation-associated genes, suggesting that the cells matured more quickly in LEO. This study is continuing with several more missions in order to understand the mechanisms underlying accelerated maturation and to investigate other neurological diseases. Our goal is to make use of the opportunity to study neural cells in LEO to better understand and treat neurodegenerative disease on Earth and to help ameliorate potentially adverse neurological effects of space travel.
Keywords: Parkinson’s disease; induced pluripotent stem cells; microglia; microgravity; multiple sclerosis; neurons; organoids.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
The authors declared no potential conflicts of interests.
Figures




References
-
- Roy-O’Reilly M, Mulavara A, Williams T.. A review of alterations to the brain during spaceflight and the potential relevance to crew in long-duration space exploration. npj Microgravity. 2021;7(1):5. https://doi.org/ 10.1038/s41526-021-00133-z - DOI - PMC - PubMed
-
- Clayton BLL, Barbar L, Sapar M, et al. Patient iPSC models reveal glia-intrinsic phenotypes in multiple sclerosis. Cell Stem Cell. 2024. https://doi.org/ 10.1016/j.stem.2024.08.002 - DOI - PMC - PubMed
-
- Paull D, Sevilla A, Zhou H, et al. Automated, high-throughput derivation, characterization and differentiation of induced pluripotent stem cells. Nat Methods. 2015;12(9):885-892. https://doi.org/ 10.1038/nmeth.3507 - DOI - PubMed
-
- Hills R, Mossman JA, Bratt-Leal AM, et al. Neurite outgrowth and gene expression profile correlate with efficacy of human induced pluripotent stem cell-derived dopamine neuron grafts. Stem Cells Dev. 2023;32(13-14):387-397. https://doi.org/ 10.1089/scd.2023.0043 - DOI - PMC - PubMed
-
- Bhutani K, Nazor KL, Williams R, et al. Whole-genome mutational burden analysis of three pluripotency induction methods. Nat Commun. 2016;7(19):10536. https://doi.org/ 10.1038/ncomms10536 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

