Pharmacological inhibition of histamine N-methyltransferase extends wakefulness and suppresses cataplexy in a mouse model of narcolepsy
- PMID: 39441998
- PMCID: PMC12477117
- DOI: 10.1093/sleep/zsae244
Pharmacological inhibition of histamine N-methyltransferase extends wakefulness and suppresses cataplexy in a mouse model of narcolepsy
Abstract
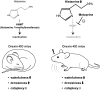
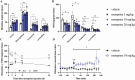
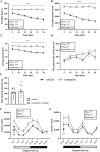
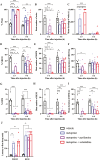
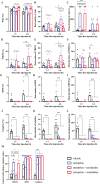
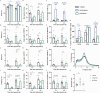
Histamine, a neurotransmitter, plays a predominant role in maintaining wakefulness. Furthermore, our previous studies showed that histamine N-methyltransferase (HNMT), a histamine-metabolizing enzyme, is important for regulating brain histamine concentration. However, the effects of pharmacological HNMT inhibition on mouse behavior, including the sleep-wake cycle and cataplexy, in a mouse model of narcolepsy have not yet been investigated. In the present study, we investigated the effects of metoprine, an HNMT inhibitor with high blood-brain barrier permeability, in wild-type (WT) and orexin-deficient (OxKO) narcoleptic mice. Metoprine increased brain histamine concentration in a time- and dose-dependent manner without affecting peripheral histamine concentrations. Behavioral tests showed that metoprine increased locomotor activity in both novel and familiar environments, but did not alter anxiety-like behavior. Sleep analysis showed that metoprine increased wakefulness and decreased non-rapid eye movement (NREM) sleep through the activation of the histamine H1 receptor (H1R) in WT mice. In contrast, the reduction of rapid eye movement (REM) sleep by metoprine occurred independent of H1R. In OxKO mice, metoprine was found to prolong wakefulness and robustly suppress cataplexy. In addition, metoprine has a greater therapeutic effect on cataplexy than pitolisant, which induces histamine release in the brain and has been approved for patients with narcolepsy. These data demonstrate that HNMT inhibition has a strong effect on wakefulness, demonstrating therapeutic potential against cataplexy in narcolepsy.
Keywords: histamine; histamine N-methyl transferase; metoprine; narcolepsy; wakefulness.
© The Author(s) 2024. Published by Oxford University Press on behalf of Sleep Research Society. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Figures
References
-
- Yoshikawa T, Naganuma F, Iida T, et al. Molecular mechanism of histamine clearance by primary human astrocytes. Glia. 2013;61(6):905–916. doi: https://doi.org/ 10.1002/glia.22484 - DOI - PubMed
-
- Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4(2):121–130. doi: https://doi.org/ 10.1038/nrn1034 - DOI - PubMed
-
- Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88(3):1183–1241. doi: https://doi.org/ 10.1152/physrev.00043.2007 - DOI - PubMed
-
- Naganuma F, Nakamura T, Kuroyanagi H, et al. Chemogenetic modulation of histaminergic neurons in the tuberomamillary nucleus alters territorial aggression and wakefulness. Sci Rep. 2021;11(1):17935. doi: https://doi.org/ 10.1038/s41598-021-95497-3 - DOI - PMC - PubMed
-
- Thakkar MM. Histamine in the regulation of wakefulness. Sleep Med Rev. 2011;15(1):65–74. doi: https://doi.org/ 10.1016/j.smrv.2010.06.004 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

