Cilgavimab and tixagevimab as pre-exposure prophylaxis in vaccine non-responder kidney transplant recipients during a period of prevalent SARS-CoV-2 BA.2 and BA.4/5 variants-a prospective cohort study (RESCUE-TX)
- PMID: 39442367
- PMCID: PMC11539723
- DOI: 10.1016/j.ebiom.2024.105417
Cilgavimab and tixagevimab as pre-exposure prophylaxis in vaccine non-responder kidney transplant recipients during a period of prevalent SARS-CoV-2 BA.2 and BA.4/5 variants-a prospective cohort study (RESCUE-TX)
Abstract
Background: The response to severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) vaccination is severely impaired in patients on maintenance immunosuppression after kidney transplantation.
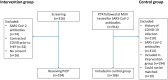
Methods: We conducted a prospective cohort study of 194 kidney transplant recipients (KTR) who exhibited no response to SARS-CoV-2 vaccinations (i.e., SARS-CoV-2 spike protein antibodies ≤264 U/mL) and had no prior documented infection. Patients received 300 mg of cilgavimab/tixagevimab as SARS-CoV-2 pre-exposure prophylaxis (PrEP) between March 4, 2022, and May 3, 2022 and were contrasted to a matched cohort of 186 KTRs also without immunization again defined as SARS-CoV-2 spike protein antibodies ≤264 U/mL and no documented prior infection. The primary outcome was the serum kinetics of cilgavimab/tixagevimab, the secondary endpoints were time to SARS-CoV-2 breakthrough infection, severity of disease and variant specific live viral in vitro neutralization tests of patient sera.
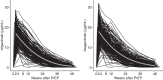
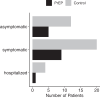
Findings: Longitudinal serum level monitoring showed a half-life of 91 days for both antibodies (95% CI 86-95 days for cilgavimab and 85-96 days for tixagevimab) in KTRs. In vitro neutralization tests showed effectiveness against the BA.2 omicron subvariant but not BA.5. The cumulative incidence of SARS-CoV-2 infections until May 15, 2022, (BA.2 dominance) was 15/194 vs 36/186 in the PrEP and control group respectively (OR = 0.35, 95% CI 0.18-0.66) but was not different thereafter (BA.4/5 dominance). The number of severe infections during the BA.2 period was lower in the prophylaxis than in the control group (OR = 0.37, 95% CI 0.17-0.79).
Interpretation: This study showed that SARS-CoV-2 PrEP with cilgavimab/tixagevimab demonstrated clinical effectiveness against variants that are neutralised (BA.2) but not against BA.4/5.
Funding: This study was funded by the Medical University of Vienna and an unrestricted grant from AstraZeneca (ESR-21-21585).
Keywords: COVID-19; Cilgavimab/tixagevimab; Kidney transplant; Vaccine non-responder.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests “The authors declare no competing interests for this manuscript”
Figures



References
-
- Derosa L., Melenotte C., Griscelli F., et al. The immuno-oncological challenge of COVID-19. Nat Cancer. 2020;1(10):946–964. - PubMed
-
- Bonelli M.M., Mrak D., Perkmann T., Haslacher H., Aletaha D. SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response. Ann Rheum Dis. 2021;80(10):1355–1356. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

