Overexpression of bacterial γ-glutamylcysteine synthetase increases toxic metal(loid)s tolerance and accumulation in Crambe abyssinica
- PMID: 39443376
- PMCID: PMC11660394
- DOI: 10.1007/s00299-024-03351-3
Overexpression of bacterial γ-glutamylcysteine synthetase increases toxic metal(loid)s tolerance and accumulation in Crambe abyssinica
Abstract
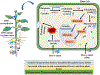
Transgenic Crambe abyssinica lines overexpressing γ-ECS significantly enhance tolerance to and accumulation of toxic metal(loid)s, improving phytoremediation potential and offering an effective solution for contaminated soil management. Phytoremediation is an attractive environmental-friendly technology to remove metal(loid)s from contaminated soils and water. However, tolerance to toxic metals in plants is a critical limiting factor. Transgenic Crambe abyssinica lines were developed that overexpress the bacterial γ-glutamylcysteine synthetase (γ-ECS) gene to increase the levels of non-protein thiol peptides such as γ-glutamylcysteine (γ-EC), glutathione (GSH), and phytochelatins (PCs) that mediate metal(loid)s detoxification. The present study investigated the effect of γ-ECS overexpression on the tolerance to and accumulation of toxic As, Cd, Pb, Hg, and Cr supplied individually or as a mixture of metals. Compared to wild-type plants, γ-ECS transgenics (γ-ECS1-8 and γ-ECS16-5) exhibited a significantly higher capacity to tolerate and accumulate these elements in aboveground tissues, i.e., 76-154% As, 200-254% Cd, 37-48% Hg, 26-69% Pb, and 39-46% Cr, when supplied individually. This is attributable to enhanced production of GSH (82-159% and 75-87%) and PC2 (27-33% and 37-65%) as compared to WT plants under AsV and Cd exposure, respectively. The levels of Cys and γ-EC were also increased by 56-67% and 450-794% in the overexpression lines compared to WT plants under non-stress conditions, respectively. This likely enhanced the metabolic pathway associated with GSH biosynthesis, leading to the ultimate synthesis of PCs, which detoxify toxic metal(loid)s through chelation. These findings demonstrate that γ-ECS overexpressing Crambe lines can be used for the enhanced phytoremediation of toxic metals and metalloids from contaminated soils.
Keywords: Crambe abyssinica; Glutamylcysteine synthetase; Heavy metals; Metal tolerance; Overexpression; Phytoremediation.
© 2024. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Declaration
The author has no conflicts of interest to declare.
Figures







References
-
- Almotairy H (2024) Mitigating Metal/Metalloid Stress in Crops: Strategies for Sustainable Agricultural Resilience. DOI: 10.5772/intechopen.115044 - DOI
-
- Anon (1993) Crambe Production and Utilization. BioOptions, Newsletter of the Centre for Alternative Plant and Animal Products. University of Minnesota, Winter, 1–3
-
- Artus NN (2006) Arsenic and cadmium phytoextraction potential of crambe compared with Indian mustard. Journal of plant nutrition 29(4): 667–679. 10.1080/01904160600564444 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

