Effectiveness, Safety, and Patterns of Real-World Isavuconazole Use in Europe (2015-2019)
- PMID: 39443403
- PMCID: PMC11582280
- DOI: 10.1007/s40121-024-01064-4
Effectiveness, Safety, and Patterns of Real-World Isavuconazole Use in Europe (2015-2019)
Abstract
Introduction: Real-world data from multinational observational studies are required to better understand the role and performance of isavuconazole in real-world practice in Europe.
Methods: A retrospective medical record review was conducted at 16 sites in Europe (France, Germany, Italy, Spain, and the United Kingdom). Eligible records were from patients aged ≥ 18 years at the time of isavuconazole initiation and received at least one dose of isavuconazole for suspected or confirmed invasive aspergillosis (IA) or invasive mucormycosis (IM) during the eligibility period (October 15, 2015 to June 30, 2019). Data were descriptively analysed. Success rates, overall survival, and times to these events were descriptively analysed.
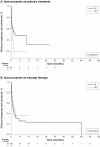
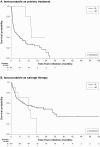
Results: Data were abstracted from 218 patients (201, IA; 17, IM) who received isavuconazole as monotherapy (initiated as infusion, 52%; oral, 46%). Isavuconazole was initiated as primary therapy in 92 patients (42.2%) and salvage therapy in 121 patients (55.5%) (unknown for five patients). Mean (standard deviation) age was 56.8 (15.6) years, 66% were men and 62% had at least three comorbidities, most frequently haematologic malignancy (62%). Estimated clinical response rate at week 24 was 54.5% (95% confidence interval [CI], 38.2-66.5%) for primary treatment and 73.5% (95% CI, 62.7-81.1%) for salvage therapy. Overall, 45 patients (21%) experienced at least one adverse event (AE). Serious AEs were experienced by 37 patients (17%), with seven related to isavuconazole; five patients (2.3%) discontinued isavuconazole monotherapy due to the serious AE. A total of 137 patients (63%) died, with 17 deaths (12.4%) related to their invasive fungal infection, 11 of whom initiated isavuconazole as salvage therapy.
Conclusions: This study adds to the growing body of evidence that whether used as first-line therapy or after the failure of other antifungal therapies, isavuconazole appears to have a promising clinical response and a good safety profile as an antifungal agent in patients with varied underlying conditions.
Trial registration: ClinicalTrials.gov NCT04550936.
Keywords: Invasive aspergillosis; Invasive mucormycosis; Isavuconazole; Real-world evidence.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Carolina Garcia-Vidal has received grants and honoraria for talks from MSD, Gilead Sciences Inc., Pfizer Inc., Janssen, Novartis, Eli Lilly, Mundipharma, and Shionogi. Carolina Garcia-Vidal has also received a national research grant from the Instituto de Salud Carlos III. David A Enoch has participated in advisory boards for Mundipharma/Napp, Pfizer Inc., and MSD and has received honoraria for consulting services to Tillots Pharmaceuticals and Pfizer Inc. Dionysios Neofytos has received research support from MSD and Pfizer Inc. and consulting fees from MSD, Pfizer Inc., Basilea, Takeda, and Gilead Sciences Inc. Katherine Houghton and Maria Jiminez are salaried employees of RTI Health Solutions. RTI Health Solutions received funding from Pfizer Inc. for the conduct of this research and medical writing services. Oliver Cornely has received consulting fees and payment or honoraria from Abbott, AbbVie, Akademie für Infektionsmedizin, Al-Jazeera Pharmaceuticals/Hikma, Amedes, AstraZeneca, Aicuris, Basilia, Biocon, Cidara, Seqirus, Deutscher Ärzteverlag, Gilead Sciences Inc., GSK, IQVIA, Janssen, Grupo Biotoscana/United Medical/Knight, Ipsen Pharma, Medscape/WebMD, MedUpdate, MSD, Moderna, Mundipharma, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Munipharma, Noscendo, Noxxon, Octopharma, Paul-Martini-Stiftung, Pardes, Partner Therapeutics, Pfizer Inc., PSI, Sandoz, Scynexis, Seres, Shionogi, streamedup!, Touch Independent, and Vitis. OC has also participated on advisory boards for Boston Strategic Partners, Cidara, IQVIA, Janssen, MedPace, PSI, Pulmocide, Shionogi, and The Prime Meridian Group. Edward Broughton, Lili Jiang, Maria Lavinea Novis de Figueiredo Valente, and Maria Fernandez are shareholders in Pfizer Inc. Tobias Lahmer has received travel grants and lecture fees from Pfizer Inc., Gilead Sciences Inc., and MSD. Antonio Pagliuca, Beate Gruener, Raoul Herbrecht, Olivier Lortholary, and Cléa Melenotte have no conflicts of interest to declare. Ethical Approval: Ethical approval or waivers were received in each country. In France, a certificate of compliance with MR-004 was obtained. In Germany, a waiver was received from the primary site (Cologne; application number 21–1325). In Italy and Spain, ethics committee approval was received at the primary sites (Italy: University of Turin, Reference ID: 441/2021; Spain: Hospital Clinic Barcelona, Reference ID: G-08431173). In the UK, ethical approval was received from the London—Fulham Research Ethics Committee (Reference ID: 20/PR/0939).
Figures
References
Associated data
LinkOut - more resources
Full Text Sources