Enhanced mGluR5 intracellular activity causes psychiatric alterations in Niemann Pick type C disease
- PMID: 39443481
- PMCID: PMC11499878
- DOI: 10.1038/s41419-024-07158-8
Enhanced mGluR5 intracellular activity causes psychiatric alterations in Niemann Pick type C disease
Abstract
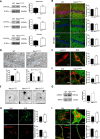
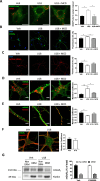
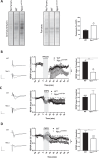
Niemann-Pick disease Type C (NPC) is caused by mutations in the cholesterol transport protein NPC1 leading to the endolysosomal accumulation of the lipid and to psychiatric alterations. Using an NPC mouse model (Npc1nmf164) we show aberrant mGluR5 lysosomal accumulation and reduction at plasma membrane in NPC1 deficient neurons. This phenotype was induced in wild-type (wt) neurons by genetic and pharmacological NPC1 silencing. Extraction of cholesterol normalized mGluR5 distribution in NPC1-deficient neurons. Intracellular accumulation of mGluR5 was functionally active leading to enhanced mGluR-dependent long-term depression (mGluR-LTD) in Npc1nmf164 hippocampal slices. mGluR-LTD was lower or higher in Npc1nmf164 slices compared with wt when stimulated with non-membrane-permeable or membrane-permeable mGluR5 agonists, respectively. Oral treatment with the mGluR5 antagonist 2-chloro-4-((2,5-dimethyl-1-(4-(trifluoromethoxy)phenyl)-1H-imidazol-4-yl)ethynyl)pyridine (CTEP) reduced mGluR-LTD and ameliorated psychiatric anomalies in the Npc1nmf164 mice. Increased neuronal mGluR5 levels were found in an NPC patient. These results implicate mGluR5 alterations in NPC psychiatric condition and provide a new therapeutic strategy that might help patients suffering from this devastating disease.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Patterson MC, Hendriksz CJ, Walterfang M, Sedel F, Vanier MT, Wijburg F Recommendations for the diagnosis and management of Niemann-Pick disease type C: an update. Mol Genet Metab. 2012;106:330–44. - PubMed
-
- Karten B, Campenot RB, Vance DE, Vance JE The Niemann-Pick C1 protein in recycling endosomes of presynaptic nerve terminals. J Lipid Res. 2006;47:504–14. - PubMed
-
- Yadid G, Sotnik-Barkai I, Tornatore C, Baker-Cairns B, Harvey-White J, Pentchev PG, et al. Neurochemical alterations in the cerebellum of a murine model of Niemann-Pick type C disease. Brain Res. 1998;799:250–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

