Effects of coagulation factors on bone cells and consequences of their absence in haemophilia a patients
- PMID: 39443571
- PMCID: PMC11499919
- DOI: 10.1038/s41598-024-75747-w
Effects of coagulation factors on bone cells and consequences of their absence in haemophilia a patients
Abstract
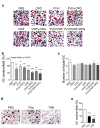
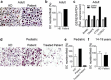
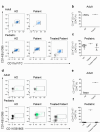
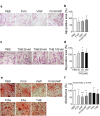
Haemophilia is associated with reduced bone mass and mineral density. Due to the rarity of the disease and the heterogeneity among the studies, the pathogenesis of bone loss is still under investigation. We studied the effects of coagulation factors on bone cells and characterized in a pilot study the osteoclastogenic potential of patients' osteoclast precursors. To evaluate the effect of coagulation factors on osteoclasts, we treated Healthy Donor-Peripheral Blood Mononuclear Cells (HD-PBMC) with Factor VIII (FVIII), von Willebrand Factor (VWF), FVIII/VWF complex, activated Factor IX (FIXa), activated Factor X (FXa) and Thrombin (THB). FVIII, VWF, FVIII/VWF, FXa and THB treatments reduced osteoclast differentiation of HD-PBMC and VWF affected also bone resorption. Interestingly, PBMC isolated from patients with moderate/severe haemophilia showed an increased osteoclastogenic potential due to the alteration of osteoclast precursors. Moreover, increased expression of genes involved in osteoclast differentiation/activity was revealed in osteoclasts of an adult patient with moderate haemophilia. Control osteoblasts treated with the coagulation factors showed that FVIII and VWF reduced ALP positivity; the opposite effect was observed following THB treatment. Moreover, FVIII, VWF and FVIII/VWF reduced mineralization ability. These results could be important to understand how coagulation factors deficiency influences bone remodeling activity in haemophilia.
Keywords: Bone diseases; Coagulation factors; Haemophilia A; Inherited coagulation disorders; Rare disease.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Escobar, M., Sallah, S. & Hemophilia A and hemophilia B: focus on arthropathy and variables affecting bleeding severity and prophylaxis. J. Thromb. Haemost. 11, 1449–1453. 10.1111/jth.12317 (2013). - PubMed
-
- Rodriguez-Merchan, E. C. et al. Joint protection in haemophilia. Haemophilia. 17 (Suppl 2), 1–23. 10.1111/j.1365-2516.2011.02615.x (2011). - PubMed
-
- Stephensen, D. & Rodriguez-Merchan, E. C. Orthopaedic co-morbidities in the elderly haemophilia population: a review. Haemophilia. 19, 166–173. 10.1111/hae.12006 (2013). - PubMed
-
- Rodriguez-Merchan, E. C. Hemophilic arthropathy: a teaching approach devoted to hemophilia treaters in under-development countries. Expert Rev. Hematol.14, 887–896. 10.1080/17474086.2021.1977118 (2021). - PubMed
-
- Samuelson Bannow, B. et al. Long-established role in haemophilia A and emerging evidence beyond haemostasis. Blood Rev.35, 43–50. 10.1016/j.blre.2019.03.002 (2019). Factor VIII. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

