Anti-IL-1RAP scFv-mSA-S19-TAT fusion carrier as a multifunctional platform for versatile delivery of biotinylated payloads to myeloid leukemia cells
- PMID: 39443595
- PMCID: PMC11500005
- DOI: 10.1038/s41598-024-76851-7
Anti-IL-1RAP scFv-mSA-S19-TAT fusion carrier as a multifunctional platform for versatile delivery of biotinylated payloads to myeloid leukemia cells
Abstract
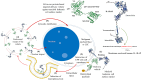
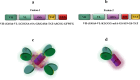
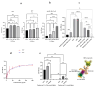
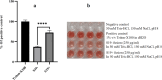
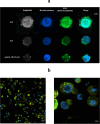
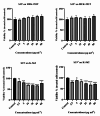
Acute myeloid leukemia (AML) is an aggressive blood cancer with frequently poor clinical outcomes. This heterogeneous malignancy encompasses genetically, molecularly, and even clinically different subgroups. This makes it difficult to develop therapeutic agents that are effective for all subtypes of the disease. Therefore, a selective, universal, and adaptable delivery platform capable of carrying various types of anti-neoplastic agents is an unmet requirement in this area. Two multifunctional fusion proteins were designed for the delivery of biotinylated cargoes to human myeloid leukemia cells by fusing an anti-IL-1RAP single-chain antibody with streptavidin (tetramer or monomer), a cell-penetrating peptide (CPP), and an endosomolytic peptide in a single biomacromolecule. The designed fusions were analyzed primarily in silico, and the biofunctionality of the selected fusion was fully characterized via several binding assays, hemolysis assay, confocal microscopy and cell cytotoxicity assay after production via the Escherichia coli (E. coli) system. The refolded protein exhibited desirable binding activity to leukemic cells, pure antigen and biotinylated BSA. Further analyses revealed efficient cellular uptake, endosomolytic activity, and nuclear penetration without any detectable cytotoxicity toward normal epithelial cells. The described platform seems to have great potential for targeted delivery of different therapeutics to malignant myeloid cells.
Keywords: Bispecific fusion protein; Cell-penetrating peptide; Endosomal escape; Myeloid leukemia; Protein-based delivery vehicle.
© 2024. The Author(s).
Conflict of interest statement
All benefits, profits, and intellectual rights associated with the possible patenting of this research belong exclusively to the first author, the corresponding author, and Pasteur Institute of Iran. There is no conflict of interest by the other authors.
Figures


Similar articles
-
Bacterial production and biophysical characterization of a hard-to-fold scFv against myeloid leukemia cell surface marker, IL-1RAP.Mol Biol Rep. 2023 Feb;50(2):1191-1202. doi: 10.1007/s11033-022-07972-3. Epub 2022 Nov 26. Mol Biol Rep. 2023. PMID: 36435922
-
A TAT-streptavidin fusion protein directs uptake of biotinylated cargo into mammalian cells.Protein Eng Des Sel. 2005 Mar;18(3):147-52. doi: 10.1093/protein/gzi014. Epub 2005 Apr 8. Protein Eng Des Sel. 2005. PMID: 15820981
-
Functionalized nanospheres for targeted delivery of paclitaxel.J Control Release. 2013 Nov 10;171(3):315-21. doi: 10.1016/j.jconrel.2013.06.017. Epub 2013 Jun 20. J Control Release. 2013. PMID: 23792807
-
Bifunctional PD-1 × αCD3 × αCD33 fusion protein reverses adaptive immune escape in acute myeloid leukemia.Blood. 2018 Dec 6;132(23):2484-2494. doi: 10.1182/blood-2018-05-849802. Epub 2018 Oct 1. Blood. 2018. PMID: 30275109
-
In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides.Peptides. 2017 Jan;87:50-63. doi: 10.1016/j.peptides.2016.11.011. Epub 2016 Nov 22. Peptides. 2017. PMID: 27887988 Review.
References
-
- 1 Deschler, B. & Lübbert, M. Acute myeloid leukemia: epidemiology and etiology. Cancer Interdiscip. Int. J. Am. Cancer Soc. 107, 2099–2107. 10.1002/cncr.22233 (2006). - PubMed
-
- Shallis, R. M., Wang, R., Davidoff, A., Ma, X. & Zeidan, A. M. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 36, 70–87. 10.1016/j.blre.2019.04.005 (2019). - PubMed
-
- Southam, C. M., Craver, L. F., Dargeon, H. W. & Burchenal, J. H. A study of the natural history of acute leukemia with special reference to the duration of the disease and the occurrence of remissions. Cancer. 4, 39–59. 10.1002/1097-0142(195101)4 (1951). :1%3C39::AID-CNCR2820040105%3E3.0.CO;2-G. - PubMed
-
- Cheung, E. et al. The leukemia strikes back: a review of pathogenesis and treatment of secondary AML. Ann. Hematol. 98, 541–559. 10.1200/JCO.2014.60.0890 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

