Deficiencies in corin and atrial natriuretic peptide-mediated signaling impair endochondral ossification in bone development
- PMID: 39443661
- PMCID: PMC11500007
- DOI: 10.1038/s42003-024-07077-6
Deficiencies in corin and atrial natriuretic peptide-mediated signaling impair endochondral ossification in bone development
Abstract
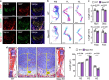
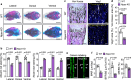
Corin is a protease that activates atrial natriuretic peptide (ANP), a hormone in cardiovascular homeostasis. Structurally, ANP is similar to C-type natriuretic peptide (CNP) crucial in bone development. Here, we examine the role of corin and ANP in chondrocyte differentiation and bone formation. We show that in Corin and Nppa (encoding ANP) knockout (KO) mice, chondrocyte differentiation is impaired, resulting in shortened limb long bones. In adult mice, Corin and Nppa deficiency impairs bone density and microarchitecture. Molecular studies in cartilages from newborn Corin and Nppa KO mice and in cultured chondrocytes indicate that corin and ANP act in chondrocytes via cGMP-dependent protein kinase G signaling to inhibit mitogen-activated protein kinase phosphorylation and stimulate glycogen synthase kinase-3β phosphorylation and β-catenin upregulation. These results indicate that corin and ANP signaling regulates chondrocyte differentiation in bone development and homeostasis, suggesting that enhancing ANP signaling may improve bone quality in patients with osteoporosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

