Anti-viral defence by an mRNA ADP-ribosyltransferase that blocks translation
- PMID: 39443800
- PMCID: PMC11618068
- DOI: 10.1038/s41586-024-08102-8
Anti-viral defence by an mRNA ADP-ribosyltransferase that blocks translation
Abstract
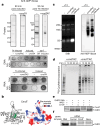
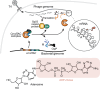
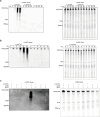
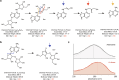
Host-pathogen conflicts are crucibles of molecular innovation1,2. Selection for immunity to pathogens has driven the evolution of sophisticated immunity mechanisms throughout biology, including in bacterial defence against bacteriophages3. Here we characterize the widely distributed anti-phage defence system CmdTAC, which provides robust defence against infection by the T-even family of phages4. Our results support a model in which CmdC detects infection by sensing viral capsid proteins, ultimately leading to the activation of a toxic ADP-ribosyltransferase effector protein, CmdT. We show that newly synthesized capsid protein triggers dissociation of the chaperone CmdC from the CmdTAC complex, leading to destabilization and degradation of the antitoxin CmdA, with consequent liberation of the CmdT ADP-ribosyltransferase. Notably, CmdT does not target a protein, DNA or structured RNA, the known targets of other ADP-ribosyltransferases. Instead, CmdT modifies the N6 position of adenine in GA dinucleotides within single-stranded RNAs, leading to arrest of mRNA translation and inhibition of viral replication. Our work reveals a novel mechanism of anti-viral defence and a previously unknown but broadly distributed class of ADP-ribosyltransferases that target mRNA.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures









References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

