Curcumin/pEGCG-encapsulated nanoparticles enhance spinal cord injury recovery by regulating CD74 to alleviate oxidative stress and inflammation
- PMID: 39443923
- PMCID: PMC11515499
- DOI: 10.1186/s12951-024-02916-4
Curcumin/pEGCG-encapsulated nanoparticles enhance spinal cord injury recovery by regulating CD74 to alleviate oxidative stress and inflammation
Abstract
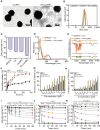
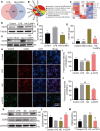
Spinal cord injury (SCI) often accompanies impairment of motor function, yet there is currently no highly effective treatment method specifically for this condition. Oxidative stress and inflammation are pivotal factors contributing to severe neurological deficits after SCI. In this study, a type of curcumin (Cur) nanoparticle (HA-CurNPs) was developed to address this challenge by alleviating oxidative stress and inflammation. Through non-covalent interactions, curcumin (Cur) and poly (-)-epigallocatechin-3-gallate (pEGCG) are co-encapsulated within hyaluronic acid (HA), resulting in nanoparticles termed HA-CurNPs. These nanoparticles gradually release curcumin and pEGCG at the SCI site. The released pEGCG and curcumin not only scavenge reactive oxygen species (ROS) and prevents apoptosis, thereby improving the neuronal microenvironment, but also regulate CD74 to promote microglial polarization toward an M2 phenotype, and inhibits M1 polarization, thereby suppressing the inflammatory response and fostering neuronal regeneration. Moreover, in vivo experiments on SCI mice demonstrate that HA-CurNPs effectively protect neuronal cells and myelin, reduce glial scar formation, thereby facilitating the repair of damaged spinal cord tissues, restoring electrical signaling at the injury site, and improving motor functions. Overall, this study demonstrates that HA-CurNPs significantly reduce oxidative stress and inflammation following SCI, markedly improving motor function in SCI mice. This provides a promising therapeutic approach for the treatment of SCI.
Keywords: CD74; Curcumin; Mice; ROS; SCI.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Microenvironment Self-Adaptive Nanomedicine Promotes Spinal Cord Repair by Suppressing Inflammation Cascade and Neural Apoptosis.Adv Mater. 2024 Dec;36(50):e2307624. doi: 10.1002/adma.202307624. Epub 2024 Oct 31. Adv Mater. 2024. PMID: 39478649
-
pH/Temperature Responsive Curcumin-Loaded Micelle Nanoparticles Promote Functional Repair after Spinal Cord Injury in Rats via Modulation of Inflammation.Tissue Eng Regen Med. 2023 Oct;20(6):879-892. doi: 10.1007/s13770-023-00567-4. Epub 2023 Aug 14. Tissue Eng Regen Med. 2023. PMID: 37580648 Free PMC article.
-
Curcumin-activated Olfactory Ensheathing Cells Improve Functional Recovery After Spinal Cord Injury by Modulating Microglia Polarization Through APOE/TREM2/NF-κB Signaling Pathway.J Neuroimmune Pharmacol. 2023 Sep;18(3):476-494. doi: 10.1007/s11481-023-10081-y. Epub 2023 Sep 2. J Neuroimmune Pharmacol. 2023. PMID: 37658943 Free PMC article.
-
A reactive oxygen species-responsive hydrogel loaded with Apelin-13 promotes the repair of spinal cord injury by regulating macrophage M1/M2 polarization and neuroinflammation.J Nanobiotechnology. 2025 Jan 10;23(1):12. doi: 10.1186/s12951-024-02978-4. J Nanobiotechnology. 2025. PMID: 39794784 Free PMC article.
-
Antioxidant nanozymes: current status and future perspectives in spinal cord injury treatments.Theranostics. 2025 May 8;15(13):6146-6183. doi: 10.7150/thno.114836. eCollection 2025. Theranostics. 2025. PMID: 40521206 Free PMC article. Review.
Cited by
-
Precision Recovery After Spinal Cord Injury: Integrating CRISPR Technologies, AI-Driven Therapeutics, Single-Cell Omics, and System Neuroregeneration.Int J Mol Sci. 2025 Jul 20;26(14):6966. doi: 10.3390/ijms26146966. Int J Mol Sci. 2025. PMID: 40725213 Free PMC article. Review.
-
Curcumin-primed milk-derived extracellular vesicles remodel hair follicle microenvironment for the treatment of androgenetic alopecia.Regen Biomater. 2025 May 30;12:rbaf051. doi: 10.1093/rb/rbaf051. eCollection 2025. Regen Biomater. 2025. PMID: 40735523 Free PMC article.
-
Pharmacological effects, molecular mechanisms and strategies to improve bioavailability of curcumin in the treatment of neurodegenerative diseases.Front Pharmacol. 2025 Jul 10;16:1625821. doi: 10.3389/fphar.2025.1625821. eCollection 2025. Front Pharmacol. 2025. PMID: 40709087 Free PMC article. Review.
-
Biomimetic elasticity compressed assembly controls rapid intracerebral drug release to reverse microglial dysfunction.Sci Adv. 2025 May 2;11(18):eadr0656. doi: 10.1126/sciadv.adr0656. Epub 2025 Apr 30. Sci Adv. 2025. PMID: 40305624 Free PMC article.
-
Inflammation-modulating polymeric nanoparticles: design strategies, mechanisms, and therapeutic applications.EBioMedicine. 2025 Aug;118:105837. doi: 10.1016/j.ebiom.2025.105837. Epub 2025 Jul 3. EBioMedicine. 2025. PMID: 40614330 Free PMC article. Review.
References
-
- Zheng J, Chen T, Wang K, Peng C, Zhao M, Xie Q, Li B, Lin H, Zhao Z, Ji Z, Tang BZ, Liao Y. Engineered Multifunctional Zinc-Organic Framework-Based Aggregation-Induced Emission Nanozyme for accelerating spinal cord Injury Recovery. ACS Nano. 2024;18:2355–69. - PubMed
MeSH terms
Substances
Grants and funding
- 2023B121203001/The Open Fund of Guangdong Provincial Key Laboratory of Laboratory of Spine and Spinal Cord Reconstruction
- Nos.32170977/National Natural Science Foundation of China
- Nos. 82102314/National Natural Science Foundation of China
- Nos.2022A1515012306/Natural Science Foundation of Guangdong Province
- Nos. 2022A1515010438/Natural Science Foundation of Guangdong Province
- Nos. JNU1AF-CFTP- 2022- a01206/Clinical Frontier Technology Program of the First Affiliated Hospital of Jinan University
- 2023A03J1024/Guangzhou Science and TechnologyPlan Projec
- 202201020018/Guangzhou Science and TechnologyPlan Project
- 2023A04J1284/Guangzhou Science and TechnologyPlan Project
- QT2024-39/Young Talent Support Project of Guangzhou Association for Science and Technology
LinkOut - more resources
Full Text Sources
Medical

