Copper hydrogen phosphate nanosheets functionalized hydrogel with tissue adhesive, antibacterial, and angiogenic capabilities for tracheal mucosal regeneration
- PMID: 39443926
- PMCID: PMC11515660
- DOI: 10.1186/s12951-024-02920-8
Copper hydrogen phosphate nanosheets functionalized hydrogel with tissue adhesive, antibacterial, and angiogenic capabilities for tracheal mucosal regeneration
Abstract
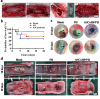
Timely and effective interventions after tracheal mucosal injury are lack in clinical practices, which elevate the risks of airway infection, tracheal cartilage deterioration, and even asphyxiated death. Herein, we proposed a biomaterial-based strategy for the repair of injured tracheal mucosal based on a copper hydrogen phosphate nanosheets (CuHP NSs) functionalized commercial hydrogel (polyethylene glycol disuccinimidyl succinate-human serum albumin, PH). Such CuHP/PH hydrogel achieved favorable injectability, stable gelation, and excellent adhesiveness within the tracheal lumen. Moreover, CuHP NSs within the CuHP/PH hydrogel effectively stimulate the proliferation and migration of endothelial/epithelial cells, enhancing angiogenesis and demonstrating excellent tissue regenerative potential. Additionally, it exhibited significant inhibitory effects on both bacteria and bacterial biofilms. More importantly, when injected injured site of tracheal mucosa under fiberoptic bronchoscopy guidance, our results demonstrated CuHP/PH hydrogel adhered tightly to the tracheal mucosa. The therapeutic effects of the CuHP/PH hydrogel were further confirmed, which significantly improved survival rates, vascular and mucosal regeneration, reduced occurrences of intraluminal infections, tracheal stenosis, and cartilage damage complications. This research presents an initial proposition outlining a strategy employing biomaterials to mitigate tracheal mucosal injury, offering novel perspectives on the treatment of mucosal injuries and other tracheal diseases.
Keywords: Angiogenesis; Antibacteria; Copper hydrogen phosphate nanosheets, Hydrogel; Tracheal mucosa repair.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Lei D, Luo B, Guo YF, Wang D, Yang H, Wang SF, Xuan HX, Shen A, Zhang Y, Liu ZH, He CL, Qing FL, Xu Y, Zhou GD. You, 4-Axis printing microfibrous tubular scaffold and tracheal cartilage application. Sci China Mater. 2019;62(12):1910–20. - DOI
-
- Deshmukh A, Jadhav S, Wadgoankar V, Takalkar U, Deshmukh H, Apsingkar P, Sonwatikar P, Antony P. Airway Management and Bronchoscopic Treatment of Subglottic and Tracheal Stenosis using Holmium laser with balloon dilatation. Indian J Otolaryngol Head Neck Surg. 2019;71(Suppl 1):453–8. - DOI - PMC - PubMed
-
- Gao W, Chen K, He W, Zhao S, Cui D, Tao C, Xu Y, Xiao X, Feng Q, Xia H. Synergistic chondrogenesis promotion and arthroscopic articular cartilage restoration via injectable dual-drug-loaded sulfated hyaluronic acid hydrogel for stem cell therapy. Compos Part B: Eng 263 (2023).
-
- Xu Y, Dai J, Zhu XS, Cao RF, Song N, Liu M, Liu XG, Zhu JJ, Pan F, Qin LL, Jiang GN, Wang HF, Yang Y. Biomimetic Trachea Engineering via a modular Ring Strategy based on bone-marrow stem cells and Atelocollagen for use in extensive Tracheal Reconstruction. Adv Mater 34(6) (2022). - PubMed
MeSH terms
Substances
Grants and funding
- 82302395, 32271386/the National Natural Science Foundation of China
- 22ZR1452100, 22YF1437400/the Natural Science Foundation of Shanghai
- 22ZR1452100, 22YF1437400/the Natural Science Foundation of Shanghai
- 2023QNRC001/the Young Elite Scientists Sponsorship Program by CAST
- Y20220142/the Wenzhou Science and Technology Project
- Y20220142/the Wenzhou Science and Technology Project
- ZY2022028/Wenzhou Science and Technology Major Project
- ZY2022028/Wenzhou Science and Technology Major Project
- WIUCASQD2020013, WIUCASQD2021030/the seed grants from the Wenzhou Institute, University of Chinese Academy of Sciences
- WIUCASQD2020013, WIUCASQD2021030/the seed grants from the Wenzhou Institute, University of Chinese Academy of Sciences

