Seasonal stability of the rumen microbiome contributes to the adaptation patterns to extreme environmental conditions in grazing yak and cattle
- PMID: 39443951
- PMCID: PMC11515522
- DOI: 10.1186/s12915-024-02035-4
Seasonal stability of the rumen microbiome contributes to the adaptation patterns to extreme environmental conditions in grazing yak and cattle
Abstract
Background: The rumen microbiome plays an essential role in maintaining ruminants' growth and performance even under extreme environmental conditions, however, which factors influence rumen microbiome stability when ruminants are reared in such habitats throughout the year is unclear. Hence, the rumen microbiome of yak (less domesticated) and cattle (domesticated) reared on the Qinghai-Tibetan Plateau through the year were assessed to evaluate temporal changes in their composition, function, and stability.
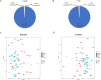
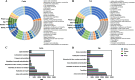
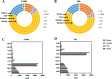
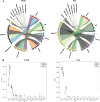
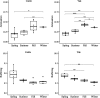
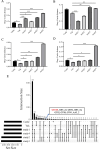
Results: Rumen fermentation characteristics and pH significantly shifted across seasons in both cattle and yak, but the patterns differed between the two ruminant species. Ruminal enzyme activity varied with season, and production of xylanase and cellulase was greater in yak compared to cattle in both fall and winter. The rumen bacterial community varied with season in both yak and cattle, with higher alpha diversity and similarity (beta diversity) in yak than cattle. The diversity indices of eukaryotic community did not change with season in both ruminant species, but higher similarity was observed in yak. In addition, the similarity of rumen microbiome functional community was higher in yak than cattle across seasons. Moreover, yak rumen microbiome encoded more genes (GH2 and GH3) related to cellulose and hemicellulose degradation compared to cattle, and a new enzyme family (GH160) gene involved in oligosaccharides was uniquely detected in yak rumen. The season affected microbiome attenuation and buffering values (stability), with higher buffering value in yak rumen microbiome than cattle. Positive correlations between antimicrobial resistance gene (dfrF) and CAZyme family (GH113) and microbiome stability were identified in yak, but such relationship was negatively correlated in cattle.
Conclusions: The findings of the potential of cellulose degradation, the relationship between rumen microbial stability and the abundance of functional genes varied differently across seasons and between yak and cattle provide insight into the mechanisms that may underpin their divergent adaptation patterns to the harsh climate of the Qinghai-Tibetan Plateau. These results lay a solid foundation for developing strategies to maintain and improve rumen microbiome stability and dig out the potential candidates for manufacturing lignocellulolytic enzymes in the yak rumen to enhance ruminants' performance under extreme environmental conditions.
Keywords: Cattle; Microbiome functionality; Rumen metagenome; Rumen microbiome stability; Yak.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Moya A, Ferrer M. Functional redundancy-induced stability of gut microbiota subjected to disturbance. Trends Microbiol. 2016;24:402–13. - PubMed
-
- Coyte KZ, Schluter J, Foster KR. The ecology of the microbiome: networks, competition, and stability. Science. 2015;350:663–6. - PubMed
-
- Pan F, Xu X, Zhang L, Luo H, Chen Y, Long L, et al. Correction: Dietary riboflavin deficiency induces genomic instability of esophageal squamous cells that is associated with gut microbiota dysbiosis in rats. Food Funct. 2020;11:10979. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

