Impairment of spinal CSF flow precedes immune cell infiltration in an active EAE model
- PMID: 39444001
- PMCID: PMC11520187
- DOI: 10.1186/s12974-024-03247-9
Impairment of spinal CSF flow precedes immune cell infiltration in an active EAE model
Abstract
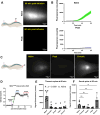
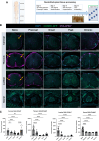
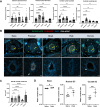
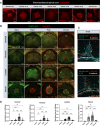
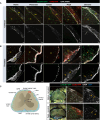
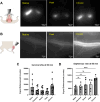
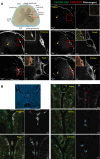
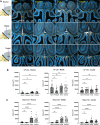
Accumulation of immune cells and proteins in the subarachnoid space (SAS) is found during multiple sclerosis and in the animal model experimental autoimmune encephalomyelitis (EAE). Whether the flow of cerebrospinal fluid (CSF) along the SAS of the spinal cord is impacted is yet unknown. Combining intravital near-infrared (NIR) imaging with histopathological analyses, we observed a significantly impaired bulk flow of CSF tracers within the SAS of the spinal cord prior to EAE onset, which persisted until peak stage and was only partially recovered during chronic disease. The impairment of spinal CSF flow coincided with the appearance of fibrin aggregates in the SAS, however, it preceded immune cell infiltration and breakdown of the glia limitans superficialis. Conversely, cranial CSF efflux to cervical lymph nodes was not altered during the disease course. Our study highlights an early and persistent impairment of spinal CSF flow and suggests it as a sensitive imaging biomarker for pathological changes within the leptomeninges.
Keywords: CSF flow; Cerebrospinal fluid; Experimental autoimmune encephalomyelitis; Glia limitans; Multiple sclerosis; Neuroinflammation; Subarachnoid space.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–69. - PubMed
-
- Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, Reynolds R, Aloisi F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–104. - PubMed
-
- Howell OW, Reeves CA, Nicholas R, Carassiti D, Radotra B, Gentleman SM, Serafini B, Aloisi F, Roncaroli F, Magliozzi R, Reynolds R. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134:2755–71. - PubMed
-
- Magliozzi R, Howell OW, Calabrese M, Reynolds R. Meningeal inflammation as a driver of cortical grey matter pathology and clinical progression in multiple sclerosis. Nat Rev Neurol. 2023;19:461–76. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

