Revisiting Virchow's triad: exploring the cellular and molecular alterations in cerebral venous congestion
- PMID: 39444013
- PMCID: PMC11515517
- DOI: 10.1186/s13578-024-01314-5
Revisiting Virchow's triad: exploring the cellular and molecular alterations in cerebral venous congestion
Abstract
Background: Cerebral venous thrombosis (CVT) is a rare but serious condition that can lead to significant morbidity and mortality. Virchow's triad elucidates the role of blood hypercoagulability, blood flow dynamics, and endothelial damage in the pathogenesis of CVT. Cerebral venous congestion (CVC) increases the risk of cerebral venous sinus thrombosis and can lead to recurrent episodes and residual symptoms. However, the precise mechanism by which blood congestion leads to thrombosis remains unclear. Our objective was to investigate the cellular and molecular alterations linked to CVC through analysis of the pathological morphology of venous sinus endothelial cells and transcriptomic profiling.
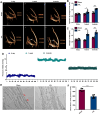
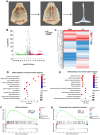
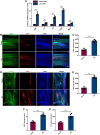
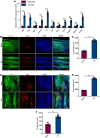
Results: This study demonstrated a remarkable correlation between CVC and the phenotypic transformation of endothelial cells from an anticoagulant to a procoagulant state. The findings revealed that cerebral venous stasis results in tortuous dilatation of the venous sinuses, with slow blood flow and elevated pressure in the sinuses and damaged endothelial cells of the retroglenoid and internal jugular vein ligation (JVL) rat model. Mechanistically, analysis of transcriptomic results of cerebral venous sinus endothelial cells showed significant activation of platelet activation, complement and coagulation cascades pathway in the JVL rats. Furthermore, the expression of von Willebrand factor (vWF) and coagulation factor VIII (F8) in the complement and coagulation cascades and Fgg and F2 in the platelet activation was increased in the cerebral venous sinuses of JVL rats than in sham rats, suggesting that endothelial cell injury in the venous sinus induced by CVC has a prothrombotic effect. In addition, endothelial cell damage accelerates coagulation and promotes platelet activation. Significantly, the concentrations of vWF, F2 and F8 in venous sinus blood of patients with internal jugular vein stenosis were higher than in their peripheral blood.
Conclusion: Collectively, our data suggest that CVC can induce endothelial cell damage, which then exhibits a procoagulant phenotype and ultimately increases the risk of CVT. This research contributes to our understanding of the pathophysiology of CVC associated with procoagulant factors and reexamines the components of Virchow's triad in the context of CVC.
Keywords: CVT; Cerebral venous congestion; Endothelial injury; Stroke; Virchow’s triad.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures
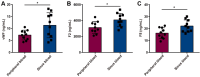
Similar articles
-
Procoagulant activity in hemostasis and thrombosis: Virchow's triad revisited.Anesth Analg. 2012 Feb;114(2):275-85. doi: 10.1213/ANE.0b013e31823a088c. Epub 2011 Nov 21. Anesth Analg. 2012. PMID: 22104070 Free PMC article. Review.
-
Thrombosis in Coronavirus disease 2019 (COVID-19) through the prism of Virchow's triad.Clin Rheumatol. 2020 Sep;39(9):2529-2543. doi: 10.1007/s10067-020-05275-1. Epub 2020 Jul 11. Clin Rheumatol. 2020. PMID: 32654082 Free PMC article. Review.
-
Pathogenesis of venous thrombosis.Chest. 1992 Dec;102(6 Suppl):640S-644S. doi: 10.1378/chest.102.6_supplement.640s. Chest. 1992. PMID: 1451539 Review.
-
Dural Venous Sinus Thrombosis: A Rare Cause of Intracranial Hemorrhage.Cureus. 2024 Nov 14;16(11):e73693. doi: 10.7759/cureus.73693. eCollection 2024 Nov. Cureus. 2024. PMID: 39677148 Free PMC article.
-
Microengineered Human Vein-Chip Recreates Venous Valve Architecture and Its Contribution to Thrombosis.Small. 2020 Dec;16(49):e2003401. doi: 10.1002/smll.202003401. Epub 2020 Nov 17. Small. 2020. PMID: 33205630 Free PMC article.
Cited by
-
Immune checkpoint inhibitors and cardiovascular toxicity: immunology, pathophysiology, diagnosis, and management.J Thromb Thrombolysis. 2025 Jul 17. doi: 10.1007/s11239-025-03146-7. Online ahead of print. J Thromb Thrombolysis. 2025. PMID: 40673965 Review.
-
Molecular mechanisms of endothelial-mesenchymal transition and its pathophysiological feature in cerebrovascular disease.Cell Biosci. 2025 Apr 19;15(1):49. doi: 10.1186/s13578-025-01393-y. Cell Biosci. 2025. PMID: 40253404 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

