Infectious etiology of intussusception in Indian children less than 2 years old: a matched case-control analysis
- PMID: 39444015
- PMCID: PMC11515542
- DOI: 10.1186/s13099-024-00659-z
Infectious etiology of intussusception in Indian children less than 2 years old: a matched case-control analysis
Abstract
Background: Enteric infections are hypothesized to be associated with intussusception in children. A small increase in intussusception following rotavirus vaccination has been seen in some settings. We conducted post-marketing surveillance for intussusception following rotavirus vaccine, Rotavac introduction in India and evaluated association of intussusception with enteric pathogens.
Methods: In a case-control study nested within a large sentinel hospital-based surveillance program in India, stool samples from 272 children aged less than 2 years admitted for intussusception and 272 age-, gender- and location-matched controls were evaluated with Taqman array card based molecular assays to detect enteric viruses, bacterial enteropathogens and parasites. Matched case-control analysis with conditional logistic regression evaluated association of enteropathogens with intussusception. Population attributable fractions (PAF) were calculated for enteropathogens significantly associated with intussusception.
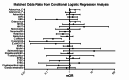
Results: The most prevalent enteropathogens in cases and controls were enteroaggregative Escherichia coli, adenovirus 40/41, adenovirus C serotypes and enteroviruses. Children with intussusception were more likely to harbor adenovirus C serotypes (adjusted odds-ratio (aOR) = 1.74; 95% confidence interval (CI) 1.06-2.87) and enteroviruses (aOR = 1.77; 95% CI 1.05-2.97) than controls. Rotavirus was not associated with increased intussusception risk. Adenovirus C (PAF = 16.9%; 95% CI 4.7% - 27.6%) and enteroviruses (PAF = 14.7%; 95% CI 4.2% - 24.1%) had the highest population attributable fraction for intussusception.
Conclusion: Adenovirus C serotypes and enteroviruses were significantly associated with intussusception in Indian children. Rotavirus was not associated with risk of intussusception.
Keywords: Adenovirus; Case-control; Intussusception; PAF; Viral pathogens.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Bines JE, Kohl KS, Forster J, Zanardi LR, Davis RL, Hansen J, et al. Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004;22(5):569–74. - PubMed
-
- Clark AD, Hasso-Agopsowicz M, Kraus MW, Stockdale LK, Sanderson CFB, Parashar UD, et al. Update on the global epidemiology of intussusception: a systematic review of incidence rates, age distributions and case-fatality ratios among children aged < 5 years, before the introduction of rotavirus vaccination. Int J Epidemiol. 2019;48(4):1316–26. - PMC - PubMed
-
- Patel MM, Lopez-Collada VR, Bulhões MM, de Oliveira LH, Márquez AB, Flannery B, et al. Intussusception Risk and Health benefits of Rotavirus Vaccination in Mexico and Brazil. N Engl J Med. 2011;364(24):2283–92. - PubMed
-
- Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R et al. Intussusception Risk after Rotavirus Vaccination in U.S. Infants. N Engl J Med New England Journal of Medicine; 1/14/2014: Massachusetts Medical Society; 2014. pp. 503 – 12. - PubMed
Grants and funding
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
- OPP1165083/Bill and Melinda Gates Foundation
LinkOut - more resources
Full Text Sources