Loss to follow-up of patients after antiviral treatment as an additional barrier to HCV elimination
- PMID: 39444018
- PMCID: PMC11515622
- DOI: 10.1186/s12916-024-03699-z
Loss to follow-up of patients after antiviral treatment as an additional barrier to HCV elimination
Erratum in
-
Correction: Loss to follow-up of patients after antiviral treatment as an additional barrier to HCV elimination.BMC Med. 2024 Nov 8;22(1):524. doi: 10.1186/s12916-024-03753-w. BMC Med. 2024. PMID: 39516834 Free PMC article. No abstract available.
Abstract
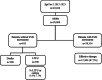
Background: Eliminating hepatitis C virus (HCV) infections is a goal set by the World Health Organization. This has become possible with the introduction of highly effective and safe direct-acting antivirals (DAA) but limitations remain due to undiagnosed HCV infections and loss of patients from the cascade of care at various stages, including those lost to follow-up (LTFU) before the assessment of the effectiveness of the therapy. The aim of our study was to determine the extent of this loss and to establish the characteristics of patients experiencing it.
Methods: Patients with chronic HCV infection from the Polish retrospective multicenter EpiTer-2 database who were treated with DAA therapies between 2015 and 2023 were included in the study.
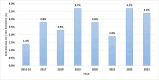
Results: In the study population of 18,968 patients, 106 had died by the end of the 12-week post-treatment follow-up period, and 509 patients did not report for evaluation of therapy effectiveness while alive and were considered LTFU. Among patients with available assessment of sustained virological response (SVR), the effectiveness of therapy was 97.5%. A significantly higher percentage of men (p<0.0001) and a lower median age (p=0.0001) were documented in LTFU compared to the group with available SVR assessment. In LTFU patients, comorbidities such as alcohol (p<0.0001) and drug addiction (p=0.0005), depression (p=0.0449) or other mental disorders (p<0.0001), and co-infection with human immunodeficiency virus (HIV) (p<0.0001) were significantly more common as compared to those with SVR assessment. They were also significantly more often infected with genotype (GT) 3, less likely to be treatment-experienced and more likely to discontinue DAA therapy.
Conclusions: In a real-world population of nearly 19,000 HCV-infected patients, we documented a 2.7% loss to follow-up rate. Independent predictors of this phenomenon were male gender, GT3 infection, HIV co-infection, alcohol addiction, mental illnesses, lack of prior antiviral treatment and discontinuation of DAA therapy.
Keywords: Direct-acting antivirals; HCV; Lost to follow-up; Treatment.
© 2024. The Author(s).
Conflict of interest statement
Zarębska-Michaluk D has acted as a speaker for AbbVie and Gilead, Brzdęk M has no conflict of interest to declare. Tronina O has no conflict of interest to declare. Janocha-Litwin J has no conflict of interest to declare. Sitko M has acted as a speaker for Gilead and AbbVie. Piekarska A has acted as a speaker and/or advisor for AbbVie, Gilead, Merck, and Roche. Klapaczyński J has acted as a speaker for Gilead and AbbVie. Parfieniuk-Kowerda A has no conflict of interest to declare. Barbara Sobala-Szczygieł has been a speaker for AbbVie and Gilead. Tudrujek-Zdunek M has no conflict of interest to declare. Lorenc B has no conflict of interest to declare. Flisiak R has acted as a speaker and/or advisor, and has received funding for clinical research from AbbVie, Gilead, Merck, and Roche.
Figures

Similar articles
-
Real-world effectiveness and safety of direct-acting antivirals in hepatitis C virus patients with mental disorders.World J Gastroenterol. 2023 Jul 7;29(25):4085-4098. doi: 10.3748/wjg.v29.i25.4085. World J Gastroenterol. 2023. PMID: 37476581 Free PMC article.
-
Direct-acting antiviral treatment of chronic HCV-infected patients on opioid substitution therapy: Still a concern in clinical practice?Addiction. 2018 May;113(5):868-882. doi: 10.1111/add.14128. Epub 2018 Jan 23. Addiction. 2018. PMID: 29359361
-
Real-world experience with direct-acting antiviral therapy in HCV-infected patients with cirrhosis and esophageal varices.Pharmacol Rep. 2024 Oct;76(5):1114-1129. doi: 10.1007/s43440-024-00639-9. Epub 2024 Aug 20. Pharmacol Rep. 2024. PMID: 39162985 Free PMC article.
-
Loss to follow-up: A significant barrier in the treatment cascade with direct-acting therapies.J Viral Hepat. 2020 Mar;27(3):243-260. doi: 10.1111/jvh.13228. Epub 2019 Dec 2. J Viral Hepat. 2020. PMID: 31664755
-
Unmet needs of chronic hepatitis C in the era of direct-acting antiviral therapy.Clin Mol Hepatol. 2020 Jul;26(3):251-260. doi: 10.3350/cmh.2020.0018. Epub 2020 Mar 19. Clin Mol Hepatol. 2020. PMID: 32188235 Free PMC article. Review.
Cited by
-
Correction: Loss to follow-up of patients after antiviral treatment as an additional barrier to HCV elimination.BMC Med. 2024 Nov 8;22(1):524. doi: 10.1186/s12916-024-03753-w. BMC Med. 2024. PMID: 39516834 Free PMC article. No abstract available.
References
-
- Razavi H, Sanchez Gonzalez Y, Yuen C, Cornberg M. Global timing of hepatitis C virus elimination in high-income countries. Liver Int. 2020;40:522–9. - PubMed
-
- Hepatitis C. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. Accessed 26 Aug 2024.
-
- World Health Organization. Global hepatitis report 2024: action for access in low-and middle-income countries. World Health Organization; 2024. https://iris.who.int/bitstream/handle/10665/376461/9789240091672-eng.pdf....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

