Humanized In Vivo Bone Tissue Engineering: In Vitro Preculture Conditions Control the Structural, Cellular, and Matrix Composition of Humanized Bone Organs
- PMID: 39444080
- PMCID: PMC11729988
- DOI: 10.1002/adhm.202401939
Humanized In Vivo Bone Tissue Engineering: In Vitro Preculture Conditions Control the Structural, Cellular, and Matrix Composition of Humanized Bone Organs
Abstract
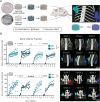
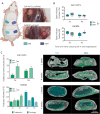
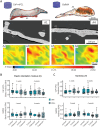
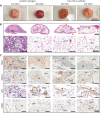
Bone tissue engineering (BTE) has long sought to elucidate the key factors controlling human/humanized bone formation for regenerative medicine and disease modeling applications, yet with no definitive answers due to the high number and co-dependency of parameters. This study aims to clarify the relative impacts of in vitro biomimetic 'preculture composition' and 'preculture duration' before in vivo implantation as key criteria for the optimization of BTE design. These parameters are directly related to in vitro osteogenic differentiation (OD) and mineralization and are being investigated across different osteoprogenitor-loaded biomaterials, specifically fibrous calcium phosphate-polycaprolactone (CaP-mPCL) scaffolds and gelatin methacryloyl (GelMA) hydrogels. The results show that OD and mineralization levels prior to implantation, enhanced by a mineralization medium supplement to the osteogenic medium (OM), significantly improve ectopic BTE outcomes, regardless of the biomaterial type. Specifically, preculture conditions are pivotal in achieving more faithful mimicry of human bone structure, cellular and extracellular matrix composition and organization, and provide control over bone marrow composition. This work emphasizes the potential of using biomimetic culture compositions, specifically the addition of a mineralization medium as a cost-effective and straightforward approach to enhance BTE outcomes, facilitating rapid development of bone models with superior quality and resemblance to native bone.
Keywords: bone structure; bone tissue engineering (BTE); extracellular matrix; humanized mouse model; in vivo bone model; mineralization; osteogenic differentiation (OD).
© 2024 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
D.W.H. is a co‐founder and shareholder of GELOMICS PTY LTD, a Brisbane‐based company developing and distributing hydrogels for 3D cell culture applications. All other authors declare no competing interests.
Figures



References
-
- Xu C., Liu Z., Chen X., Gao Y., Wang W., Zhuang X., Zhang H., Dong X., Chin. Chem. Lett. 2024, 35, 109197.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

