Mevalonate in blood and muscle: Response to atorvastatin treatment and the relationship to statin intolerance in patients with coronary heart disease
- PMID: 39444168
- PMCID: PMC11499300
- DOI: 10.1111/cts.70025
Mevalonate in blood and muscle: Response to atorvastatin treatment and the relationship to statin intolerance in patients with coronary heart disease
Abstract
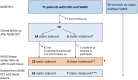
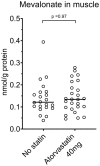
Statin-associated muscle symptoms are frequently reported and often lead to discontinuation of statin therapy with an increased risk of cardiovascular events. In vitro studies suggest that statin-mediated inhibition of the mevalonate pathway leads to muscle cell toxicity. We aimed to determine the relationship between mevalonate, LDL-cholesterol, and atorvastatin metabolites in patients with coronary heart disease and self-perceived muscle side effects. Furthermore, we assessed the correlation between mevalonate in blood and muscle and the relationship to statin intolerance due to muscle symptoms. We used blood plasma from a randomized crossover trial (n = 70) and muscle biopsies and plasma from a subgroup in a subsequent open intervention study (n = 26). Both studies tested atorvastatin 40 mg/day. Seven patients did not tolerate ≥3 statins throughout the follow-up and were classified as statin-intolerant. Mevalonate in blood plasma decreased during atorvastatin treatment (median difference -38%, range -77% to 43%, p < 0.001), whereas mevalonate in muscle tissue was not lowered (0.05%, range -47% to 145%). Mevalonate correlated poorly with LDL-cholesterol and atorvastatin metabolites (Spearman's rho -0.28 to 0.10). The statin-intolerant patients had a smaller reduction in circulating mevalonate compared with the tolerant patients; median difference -8.1 (-22 to 3.5) nmol/L versus -25 (-93 to 12) nmol/L, p = 0.028. A similar observation was made for LDL-cholesterol. Cutoffs based on these biomarkers classified >50% correctly as tolerant. Inhibition of the mevalonate pathway does not appear to be the mechanism underlying statin intolerance in the present study. Further studies of mevalonate as a biomarker for statin tolerance are needed to clarify the potential.
© 2024 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
JM reports having received modest lecture fees from Novartis, Amgen, Bohringer Ingelheim, Astra Zenika, and Bayer, outside the submitted work. NTV reports having received a modest lecture fee from Novartis, outside the submitted work. All other authors declared no competing interests in this work.
Figures


References
-
- Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111‐188. - PubMed
-
- Chowdhury R, Khan H, Heydon E, et al. Adherence to cardiovascular therapy: a meta‐analysis of prevalence and clinical consequences. Eur Heart J. 2013;34(38):2940‐2948. - PubMed
-
- Thompson PD, Taylor BA. Statin‐Associated Muscle Symptoms. Springer International Publishing: Imprint: Springer; 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

