HIV postnatal prophylaxis and infant feeding policies vary across Europe: results of a Penta survey
- PMID: 39444189
- PMCID: PMC11786621
- DOI: 10.1111/hiv.13723
HIV postnatal prophylaxis and infant feeding policies vary across Europe: results of a Penta survey
Abstract
Objectives: This survey was conducted to describe current European postnatal prophylaxis (PNP) and infant feeding policies with the aim of informing future harmonized guidelines.
Methods: A total of 32 senior clinicians with relevant expertise, working in 20 countries within the European Region, were invited to complete a REDCap questionnaire between July and September 2023.
Results: Twenty-three of the 32 invited paediatricians responded, representing 16/20 countries. There were multiple respondents from the same country for Italy (n = 5), the UK (n = 2), Germany (n = 2) and France (n = 2). All countries use risk stratification to guide PNP regimen selection. Nine out of 16 countries reported three risk categories, six out of 16 reported two, and one country reported differences in categorization. Criteria used to stratify risk varied between and within countries. For the lowest risk category, the PNP regimen reported ranged from no PNP to up to four weeks of one drug; the drug of choice reported was zidovudine, apart from one country which reported nevirapine. For the highest risk category, the most common regimen was zidovudine/lamivudine/nevirapine (20/23 respondents); regimen duration varied from two to six weeks with variation in recommended dosing. Guidelines support breastfeeding for infants born to people living with HIV in eight out of 16 countries; in the other eight, guidelines do not support/specify.
Conclusions: Guidelines and practice for PNP and infant feeding vary substantially across Europe and within some countries, reflecting the lack of robust evidence. Effort is needed to align policies and practice to reflect up-to-date knowledge to ensure the vertical transmission risk is minimized and unnecessary infant HIV testing and PNP avoided, while simultaneously supporting families to make informed decisions on infant feeding choice.
Keywords: HIV; breastfeeding; paediatrics; policy; postnatal prophylaxis; vertical transmission.
© 2024 The Author(s). HIV Medicine published by John Wiley & Sons Ltd on behalf of British HIV Association.
Conflict of interest statement
CT received funding to the Penta Foundation from ViiV Healthcare and MSD. CD received funding from ViiV to attend the IAS 2023 conference and funding from MSD for an oral presentation at a symposium 2023. PF received grants from ANRS‐MIE which were paid to his institution, personal fees from MSD France, ViiV Healthcare, Janssen Cilag, Gilead Sciences, support for attending meetings/and or travel from MSD France, Gilead Sciences and ViiV Healthcare. All other authors declare they have no competing interests.
Figures
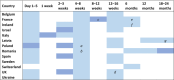
 This test will be performed only for infants classified as being at high risk of vertical transmission. a, if the infant is classified as high risk for vertical transmission, the respondent indicated that this test would instead be conducted 2 weeks after stopping presumptive therapy; b, if the infant is classified as high‐risk, the respondent indicated this test would instead be conducted at 2 weeks; c, one out of two respondents indicated testing at this time point was optional; d, one out of two respondents reported this time point to be 10–12 weeks if low‐risk, and 12 weeks if high‐risk; e, this time point was reported by one out of two respondents only; f, the respondent indicated the test at this time point would not be performed if there were two prior negative PCRs; g, the respondent indicated that testing at this timepoint would only be conducted if necessary.
This test will be performed only for infants classified as being at high risk of vertical transmission. a, if the infant is classified as high risk for vertical transmission, the respondent indicated that this test would instead be conducted 2 weeks after stopping presumptive therapy; b, if the infant is classified as high‐risk, the respondent indicated this test would instead be conducted at 2 weeks; c, one out of two respondents indicated testing at this time point was optional; d, one out of two respondents reported this time point to be 10–12 weeks if low‐risk, and 12 weeks if high‐risk; e, this time point was reported by one out of two respondents only; f, the respondent indicated the test at this time point would not be performed if there were two prior negative PCRs; g, the respondent indicated that testing at this timepoint would only be conducted if necessary.
References
-
- The integrated screening outcomes surveillance service . ISOSS HIV Report 2022. Available from: NHS infectious diseases in pregnancy screening programme, integrated screening outcomes surveillance service HIV report 2022.
-
- Illan Ramos M, Prieto Tato LM, Guillen Martin S, et al. Clinical and epidemiologic characteristics of a cohort of HIV‐infected mother‐infant pairs during 21 years. J Acquir Immune Defic Syndr. 2022;91(5):479‐484. - PubMed
-
- Peters H, Francis K, Sconza R, et al. UK mother‐to‐child HIV transmission rates continue to decline: 2012–2014. Clin Infect Dis. 2017;64(4):527‐528. - PubMed
-
- Sibiude J, Le Chenadec J, Mandelbrot L, et al. Update of perinatal human immunodeficiency virus type 1 transmission in France: zero transmission for 5482 mothers on continuous antiretroviral therapy from conception and with undetectable viral load at delivery. Clin Infect Dis. 2023;76(3):e590‐e598. - PubMed

