Rosmarinic acid promotes cartilage regeneration through Sox9 induction via NF-κB pathway inhibition in mouse osteoarthritis progression
- PMID: 39444399
- PMCID: PMC11497390
- DOI: 10.1016/j.heliyon.2024.e38936
Rosmarinic acid promotes cartilage regeneration through Sox9 induction via NF-κB pathway inhibition in mouse osteoarthritis progression
Abstract
Background: The natural polyphenolic compound known as Rosmarinic acid (RosA) can be found in various plants. Although its potential health benefits have been extensively studied, its effect on osteoarthritis (OA) progression and cartilage regeneration function still needs to be fully elucidated in OA animal models. This study elucidated the effect of RosA on OA progression and cartilage regeneration.
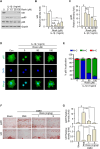
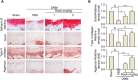
Methods: In vitro assessments were conducted using RT-PCR, qRT-PCR, Western blotting, and ELISA to measure the effects of RosA. The molecular mechanisms of RosA were determined by analyzing the translocation of p65 into the nucleus using immunocytochemistry (ICC). Histological analysis of cartilage explant was performed using alcian blue staining and immunohistochemistry (IHC). For in vivo analysis, the destabilization of the medial meniscus (DMM)-induced OA mouse model was utilized to evaluate cartilage destruction through Safranin-O staining. The expression of catabolic and anabolic factors in mice knee joints was quantified by immunohistochemistry.
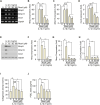
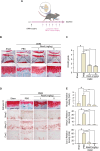
Results: The expression of catabolic factors in chondrocytes was significantly impeded by RosA. It also suppressed the NF-κB signaling pathway by decreasing phosphorylation of p65 and reducing degradation of IκB protein. In ex vivo experiments, RosA protected sulfated proteoglycan erosion triggered by IL-1β and suppressed the catabolic factors in cartilage explant. RosA treatment in animal models resulted in preventing cartilage destruction and reducing catabolic factors in the cartilage. RosA was also found to promote the expression of Sox9, Col2a1, and Acan in vitro, ex vivo, and in vivo analyses.
Conclusions: RosA attenuated the OA progression by suppressing the catabolic factors expression. These effects were facilitated through the suppression of the NF-κB signaling pathway. Additionally, it promotes cartilage regeneration by inducing anabolic factors. Therefore, RosA shows potential as an effective therapeutic agent for treating OA.
Keywords: Extracellular matrix; NF-κB; Osteoarthritis; Rosmarinic acid; Sox9.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

