Immunogenicity of concomitant SARS-CoV-2 and influenza vaccination in UK healthcare workers: a prospective longitudinal observational study
- PMID: 39444701
- PMCID: PMC11496956
- DOI: 10.1016/j.lanepe.2024.101022
Immunogenicity of concomitant SARS-CoV-2 and influenza vaccination in UK healthcare workers: a prospective longitudinal observational study
Abstract
Background: Co-administration of inactivated influenza vaccine (IIV) and SARS-CoV-2 vaccine may impact SARS-CoV-2 vaccine induced humoral immune responses. We aimed to compare IIV and SARS-CoV-2 vaccine induced cellular and humoral immune responses in those receiving concomitant vaccination to those receiving these vaccines separately.
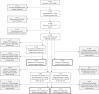
Methods: We conducted a cohort study between 29th September 2021 and 5th August 2022 in healthcare workers who worked at the local NHS trust and in the surrounding area that were vaccinated with a mRNA SARS-CoV-2 booster and cell-based IIV. We measured haemagglutination inhibition assay (HAI) titres, SARS-CoV-2 anti-spike antibody and SARS-CoV-2 ELISpot count pre-vaccination, 1-month and 6-months post-vaccination and evaluated differences by vaccine strategy.
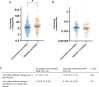
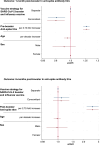
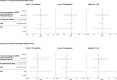
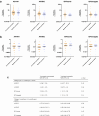
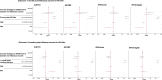
Findings: We recruited 420 participants, 234/420 (56%) were vaccinated concomitantly and 186/420 (44%) separately. The 1-month post-vaccination mean fold rise (MFR) in SARS-CoV-2 anti-spike antibodies was lower in those vaccinated concomitantly compared to separately (MFR [95% confidence interval (CI)] 9.7 [8.3, 11.4] vs 12.8 [10.3, 15.9], p = 0.04). After adjustment for age and sex, the adjusted geometric mean ratio (aGMR) remained lower for those vaccinated concomitantly compared to separately (aGMR [95% CI] 0.80 [0.70, 0.92], p = 0.001). At 6-months post-vaccination, we found no statistically significant difference in SARS-CoV-2 anti-spike antibody titres (aGMR [95% CI] 1.09 [0.87, 1.35], p = 0.45). We found no statistically significant correlation between vaccine strategy with SARS-CoV-2 ELISpot count and influenza HAI titres at 1-month and 6-months post-vaccination.
Interpretation: Our study found that concomitant vaccination with SARS-CoV-2 and IIV has no statistically significant impacts on long-term immunogenicity. Further research is required to understand the underlying mechanisms and assess the clinical significance of reduced anti-spike antibodies in those vaccinated concomitantly.
Funding: Research and Innovation (UKRI) through the COVID-19 National Core Studies Immunity (NCSi) programme (MC_PC_20060).
Keywords: Immunogenicity; Influenza; SARS-CoV-2; Vaccine co-administration.
© 2024 The Authors.
Conflict of interest statement
PM has received honoraria from Moderna, BioNTech, Gilead, AstraZeneca and GSK, support for attending meetings from AstraZeneca and has participated on an advisory board for AstraZeneca and Moderna. IB declares shares in an influenza vaccine manufacturing company. PH received an honorarium for hosting a COVID-19 webinar, on behalf of Oxford Immunotec (now Revvity) who are manufacturers of the ELISpot technology used in the manuscript. AT is an employee of Revvity. MP reports grants from UKRI-MRC for the current work and UKRI-MRC, NIHR, Sanofi, Gilead and Moderna outside the current work and has received consulting fees from QIAGEN. SGS reports consultancy or advisory role for CSL Seqirus, Novavax, Moderna, Sanofi and Evo Health. The WHO Collaborating Centre for Reference and Research on Influenza received funding from the International Federation of Pharmaceutical Manufacturers and Associations and from CSL Seqirus for the production of influenza vaccines. DP is supported by a NIHR Doctoral Research Fellowship. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Figures
References
-
- Moriyama M., Hugentobler W.J., Iwasaki A. Seasonality of respiratory viral infections. Annu Rev Virol. 2020;7(1):83–101. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

