Current and future role of MRI in the diagnosis and prognosis of multiple sclerosis
- PMID: 39444702
- PMCID: PMC11496980
- DOI: 10.1016/j.lanepe.2024.100978
Current and future role of MRI in the diagnosis and prognosis of multiple sclerosis
Abstract
In the majority of cases, multiple sclerosis (MS) is characterized by reversible episodes of neurological dysfunction, often followed by irreversible clinical disability. Accurate diagnostic criteria and prognostic markers are critical to enable early diagnosis and correctly identify patients with MS at increased risk of disease progression. The 2017 McDonald diagnostic criteria, which include magnetic resonance imaging (MRI) as a fundamental paraclinical tool, show high sensitivity and accuracy for the diagnosis of MS allowing early diagnosis and treatment. However, their inappropriate application, especially in the context of atypical clinical presentations, may increase the risk of misdiagnosis. To further improve the diagnostic process, novel imaging markers are emerging, but rigorous validation and standardization is still needed before they can be incorporated into clinical practice. This Series article discusses the current role of MRI in the diagnosis and prognosis of MS, while examining promising MRI markers, which could serve as reliable predictors of subsequent disease progression, helping to optimize the management of individual patients with MS. We also explore the potential of new technologies, such as artificial intelligence and automated quantification tools, to support clinicians in the management of patients. Yet, to ensure consistency and improvement in the use of MRI in MS diagnosis and patient follow-up, it is essential that standardized brain and spinal cord MRI protocols are applied, and that interpretation of results is performed by qualified (neuro)radiologists in all countries.
Keywords: Diagnosis; Magnetic resonance imaging; Multiple sclerosis.
© 2024 The Author(s).
Conflict of interest statement
Maria A. Rocca received consulting fees from Biogen, Bristol Myers Squibb, Eli Lilly, Janssen, Roche; and speaker honoraria from AstraZaneca, Biogen, Bristol Myers Squibb, Bromatech, Celgene, Genzyme, Horizon Therapeutics Italy, Merck Serono SpA, Novartis, Roche, Sanofi and Teva. She receives research support from the MS Society of Canada, the Italian Ministry of Health, the Italian Ministry of University and Research, and Fondazione Italiana Sclerosi Multipla. She is Associate Editor for Multiple Sclerosis and Related Disorders. Paolo Preziosa received speaker honoraria from Roche, Biogen, Novartis, Merck Serono, Bristol Myers Squibb, Genzyme, Horizon and Sanofi. He has received research support from Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla. Frederik Barkhof acts in Steering committee or Data Safety Monitoring Board member for Biogen, Merck, ATRI/ACTC and Prothena. Consultant for Roche, Celltrion, Rewind Therapeutics, Merck, IXICO, Jansen, Combinostics. Research agreements with Merck, Biogen, GE Healthcare, Roche. Co-founder and shareholder of Queen Square Analytics LTD. FB is supported by the NIHR Biomedical Research Centre at UCLH. Wallace Brownlee has received speaker honoraria and/or acted as a consultant for Biogen, Merck, Novartis, Roche, Sandoz, Sanofi and Viatris. WB is supported by the NIHR University College London Hospitals Biomedical Research Centre. Massimiliano Calabrese received speaker honoraria and travel grants from Biogen, Bristol Myers Squibb- Celgene, Sanofi-Genzyme, Merck Serono, Novartis Pharma, and Roche and received research support from the Progressive MS Alliance and Italian Minister of Health and from Biogen, Bristol Myers Squibb-Celgene, Novartis Pharma, Sanofi-Genzyme, Merck Serono, and Roche. Nicola De Stefano declared no competing interests. Cristina Granziera's employer (University Hospital Basel) has received the following fees which were used exclusively for research support: advisory boards and consultancy fees from Actelion, Novartis, Genzyme-Sanofi, GeNeuro, Hoffmann La Roche, Merck and Siemens Healthineers; speaker fees from Biogen, Hoffmann La Roche, Teva, Novartis, Janssen, Merck and Genzyme-Sanofi; and research grants from Hoffmann La Roche, GeNeuro, Genzyme, Novartis and Biogen. CG is supported by the Swiss National Fund n. PP00P3_206151, the Hasler Foundation and the Stiftung zur Förderung der gastroenterologischen und allgemeinen klinischen Forschung. Stefan Ropele declared no competing interests. Ahmed T Toosy reports receiving speaker honoraria from Merck, Bayer, Biomedia, and Serono Symposia International Foundation; receiving meeting expenses from Merck, Biogen Idec; and being the United Kingdom principal investigator for two clinical trials sponsored by MedDay Pharma. ATT is supported by recent grants from the MRC (MR/S026088/1), NIHR BRC (541/CAP/OC/818837) and RoseTrees Trust (A1332 and PGL21/10079). Àngela Vidal-Jordana has received support has received support for contracts Juan Rodes (JR16/00024) and from Fondo de Investigación en Salud (PI17/02162 and PI22/01589) from Instituto de Salud Carlos III, Spain, and has engaged in consulting and/or participated as speaker in events organized by Roche, Novartis, and Merck. Massimo Filippi is Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Neurological Sciences, and Radiology; received compensation for consulting services from Alexion, Almirall, Biogen, Merck, Novartis, Roche, Sanofi; speaking activities from Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, Janssen, Merck-Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and TEVA; participation in Advisory Boards for Alexion, Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Genzyme, Takeda; scientific direction of educational events for Biogen, Merck, Roche, Celgene, Bristol-Myers Squibb, Lilly, Novartis, Sanofi-Genzyme; he receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, the Italian Ministry of Health, the Italian Ministry of University and Research, and Fondazione Italiana Sclerosi Multipla. Massimiliano Di Filippo participated on advisory boards and steering committees for and received speaker or writing honoraria, research support and funding for travelling from Alexion, BMS, Bayer, Biogen Idec, Genzyme, Horizon, Merck, Mylan, Novartis, Roche, Siemens Healthineers, Teva and Viatris. Role of the funding source: None.
Figures
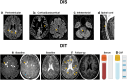
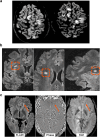
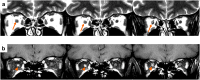
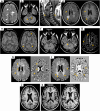
References
-
- Thompson A.J., Banwell B.L., Barkhof F., et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. - PubMed
-
- Geraldes R., Ciccarelli O., Barkhof F., et al. The current role of MRI in differentiating multiple sclerosis from its imaging mimics. Nat Rev Neurol. 2018;14(4):199–213. - PubMed
-
- Solomon A.J., Arrambide G., Brownlee W.J., et al. Differential diagnosis of suspected multiple sclerosis: an updated consensus approach. Lancet Neurol. 2023;22(8):750–768. - PubMed
-
- McDonald W.I., Compston A., Edan G., et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121–127. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

