Advances in the prerequisite and consequence of STING downstream signalosomes
- PMID: 39444795
- PMCID: PMC11495525
- DOI: 10.1515/mr-2024-0016
Advances in the prerequisite and consequence of STING downstream signalosomes
Abstract
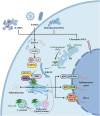
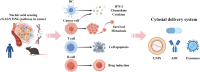
The cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway is an evolving DNA-sensing mechanism involved in innate immunity and pathogen defense that has been optimized while remaining conserved. Aside from recognizing pathogens through conserved motifs, these receptors also detect aberrant or misplaced self-molecules as possible signs of perturbed homeostasis. Upon binding external or self-derived DNA, a mobile secondary messenger 2'3'-cyclic GMP-AMP (cGAMP) is produced by cGAS and in turn activates its adapter STING in the endoplasmic reticulum (ER). Resting-state or activated STING protein is finely restricted by multiple degradation machineries. The post-translational changes of the STING protein, along with the regulatory machinery of the secret routes, limit the onset, strength and sustention of STING signal. STING experiences a conformational shift and relocates with TBK1 from the ER to perinuclear vesicles containing transcription factors, provoking the transcription activity of IRF3/IFN-I and NF-κB pathways, as well as to initiate a number of cellular processes that have been shown to alter the immune landscape in cancer, such as autophagy, NLRP3 inflammasome, ER stress, and cell death. STING signal thus serves as a potent activator for immune mobilization yet also triggers immune-mediated pathology in tissues. Recent advances have established the vital role of STING in immune surveillance as well as tumorigenic process. This review provides an overview of the disparate outcomes of cancer attributed to the actions of pleiotropic and coordinated STING downstream signalosomes, along with the underlying mechanisms of STING function in pathologies, providing therapeutic implications for new approaches in hunt for the next generation of cancer immunotherapy base on STING.
Keywords: cancer therapeutics; cellular heterogeneity; signalosomes; stimulator of interferon genes.
© 2024 the author(s), published by De Gruyter, Berlin/Boston.
Conflict of interest statement
Competing interests: Authors state no conflict of interest.
Figures
Similar articles
-
The cGAS-STING signaling in cardiovascular and metabolic diseases: Future novel target option for pharmacotherapy.Acta Pharm Sin B. 2022 Jan;12(1):50-75. doi: 10.1016/j.apsb.2021.05.011. Epub 2021 May 20. Acta Pharm Sin B. 2022. PMID: 35127372 Free PMC article. Review.
-
Interaction between the SFTSV envelope glycoprotein Gn and STING inhibits the formation of the STING-TBK1 complex and suppresses the NF-κB signaling pathway.J Virol. 2024 Mar 19;98(3):e0181523. doi: 10.1128/jvi.01815-23. Epub 2024 Feb 29. J Virol. 2024. PMID: 38421179 Free PMC article.
-
The role of cGAS-STING signalling in liver diseases.JHEP Rep. 2021 Jun 24;3(5):100324. doi: 10.1016/j.jhepr.2021.100324. eCollection 2021 Oct. JHEP Rep. 2021. PMID: 34381984 Free PMC article. Review.
-
cGAS-STING signaling pathway in intestinal homeostasis and diseases.Front Immunol. 2023 Sep 14;14:1239142. doi: 10.3389/fimmu.2023.1239142. eCollection 2023. Front Immunol. 2023. PMID: 37781354 Free PMC article. Review.
-
Role of micronucleus-activated cGAS-STING signaling in antitumor immunity.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Jan 26;53(1):25-34. doi: 10.3724/zdxbyxb-2023-0485. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38273467 Free PMC article. Review. Chinese, English.
Cited by
-
Clinical approaches to overcome PARP inhibitor resistance.Mol Cancer. 2025 May 30;24(1):156. doi: 10.1186/s12943-025-02355-1. Mol Cancer. 2025. PMID: 40442774 Free PMC article. Review.
-
Metal-organic frameworks activate the cGAS-STING pathway for cancer immunotherapy.J Nanobiotechnology. 2025 Aug 21;23(1):578. doi: 10.1186/s12951-025-03669-4. J Nanobiotechnology. 2025. PMID: 40841898 Free PMC article. Review.
-
Research and progress of cGAS/STING/NLRP3 signaling pathway: a mini review.Front Immunol. 2025 May 20;16:1594133. doi: 10.3389/fimmu.2025.1594133. eCollection 2025. Front Immunol. 2025. PMID: 40463371 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
