Clinical Analysis of 32 Cases of Subglottic Benign Airway Stenosis Treated With Montgomery T Silicone Stent
- PMID: 39444845
- PMCID: PMC11498979
- DOI: 10.1155/2024/2145560
Clinical Analysis of 32 Cases of Subglottic Benign Airway Stenosis Treated With Montgomery T Silicone Stent
Abstract
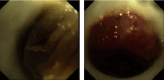
Objective: To explore the complications of long-term placement of Montgomery T silicone stent (T-tube) in the treatment of subglottic benign airway stenosis (SBAS) and the timing of successful T-tube removal. Methods: We retrospectively collected the clinical data of 32 patients with SBAS who underwent the treatment of T-tube and analyzed their placement and successful removal of the T-tube. Results: There were 22 males and 10 females, aged from 21 to 79 years (60.9 ± 13.7 years). The T-tubes were successfully placed in all 32 patients, and 6 patients (18.8%) with mild stenosis were placed by the intravenous conscious sedation. The longest follow-up period was 60.4 months, and 17 patients (53.1%) had the T-tubes for more than 12 months; 5 patients (15.6%) were changed to the tracheostomy cannula after unplanned removal of the T-tubes for various reasons; the T-tubes were successfully removed in 9 patients (28.1%), and the duration of T-tubes placement was 5.2-22.7 months (12.1 ± 6.3 months), among them anatomical stenosis in 9 patients (100%). Secretion retention was observed in 32 patients (100%), granulation tissue hyperplasia was observed in 9 patients (28.1%), and the normal ventilation was not affected in most patients by bronchoscopic treatment and follow-up; the T-tubes were removed in 3 patients due to severe complications. There was no significant difference in the incidences of secretion retention and granulation tissue hyperplasia between the time point at 1 week, 1 month, 3 months, and 12 months, p > 0.05. In patients with T-tube more than 12 months, the severity of secretion retention at 1 week, 1 month, 3 months, and 12 months was significantly different, p < 0.05, however, there was no significant difference in the severity of granulation tissue hyperplasia, p > 0.05. Conclusions: T-tube is safe and effective in the treatment of SBAS. The severity of secretion retention increased in patients with long-term placement of the T-tube. For patients with mild stenosis and anatomical stenosis, the T-tube removal can be attempted at about 1 year of follow-up.
Keywords: complication; long-term follow-up; montgomery T silicone stent; stent removal; subglottic benign airway stenosis.
Copyright © 2024 Zhenyu Yang et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

