Enhancing outcomes in extensive-stage small cell lung cancer brain metastases: a retrospective study on the synergistic effects of immune checkpoint inhibitor, brain radiotherapy, and chemotherapy
- PMID: 39444875
- PMCID: PMC11494590
- DOI: 10.21037/jtd-24-654
Enhancing outcomes in extensive-stage small cell lung cancer brain metastases: a retrospective study on the synergistic effects of immune checkpoint inhibitor, brain radiotherapy, and chemotherapy
Abstract
Background: Brain metastasis is a frequent complication in small cell lung cancer (SCLC), and there is an urgent need for new treatment modalities, given the limited success of traditional approaches. This study evaluates the combined efficacy and safety of brain radiotherapy (BRT), chemotherapy, and immune checkpoint inhibitors (ICIs) in the treatment of brain metastases in patients with extensive-stage SCLC (ES-SCLC). Additionally, it seeks to identify prognostic factors in these cases.
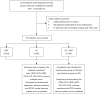
Methods: A retrospective analysis was performed on 187 patients with ES-SCLC and brain metastases treated at Zhejiang Cancer Hospital from January 2017 to October 2023. Patients were divided into three groups based on their initial treatment: BRT alone, ICI alone, and a combined ICI + BRT approach, with chemotherapy included in all regimens. Variables such as age, number of brain metastases, symptoms, comorbidities, Karnofsky Performance Status (KPS) scores, smoking history, Graded Prognostic Assessment (GPA) scores, survival time, and treatment-related adverse events (TRAEs), including hematologic and hepatic toxicities were evaluated. Prognostic factors were assessed using univariate and multivariate analyses via Cox's proportional hazards model. The study also compared outcomes and TRAEs between patients undergoing synchronous treatment (ICI and BRT within four weeks) versus those with asynchronous therapy (more than four weeks apart).
Results: Median overall survival (OS) times differed significantly across the groups: 11.6 months for BRT, 11.6 months for ICI, and 20.9 months for ICI + BRT (P<0.001). The ICI + BRT group also exhibited notably better progression-free survival (PFS) and intracranial PFS (iPFS), with medians of 12.6 and 14.9 months, respectively (P<0.001). This group demonstrated significantly improved systemic and intracranial objective response rates (ORR) and disease control rates (DCR). No significant differences in acute radiation injury rates were observed between the BRT and ICI + BRT groups. Multivariate analysis identified several factors influencing OS, including treatment regimen, number of chemotherapy and ICI cycles, presence of bone and multiple brain metastases, and antiangiogenesis therapies and extracranial radiotherapy. Both atezolizumab and serplulimab ICIs, in combination with various radiotherapy regimens [whole BRT (WBRT), WBRT with boost], were effective. Notably, asynchronous ICI and BRT treatment demonstrated advantages in PFS and iPFS over concurrent therapy, with no significant differences in other therapeutic indices or TRAE incidence rates.
Conclusions: For ES-SCLC patients with synchronous brain metastases, combined ICI and BRT, alongside chemotherapy, surpasses the efficacy of either treatment alone with manageable TRAEs. Importantly, asynchronous ICI and BRT therapy showed superior outcomes compared to synchronous treatment modalities.
Keywords: Small cell lung cancer (SCLC); brain metastasis; brain radiotherapy (BRT); immunotherapy.
2024 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-654/coif). The authors have no conflicts of interest to declare.
Figures

References
-
- Bryan S, Masoud H, Weir HK, et al. Cancer in Canada: Stage at diagnosis. Health Rep 2018;29:21-5. - PubMed
LinkOut - more resources
Full Text Sources
