Efficacy and safety of mesenchymal stem cell therapy for acute respiratory distress syndrome-a systematic review and meta-analysis
- PMID: 39444918
- PMCID: PMC11494583
- DOI: 10.21037/jtd-24-281
Efficacy and safety of mesenchymal stem cell therapy for acute respiratory distress syndrome-a systematic review and meta-analysis
Abstract
Background: Mesenchymal stem cells (MSC) therapy for acute respiratory distress syndrome (ARDS) represents a burgeoning treatment approach, supported by numerous preclinical studies confirming its efficacy. Our study aims to provide a comprehensive evaluation of both the safety and effectiveness of MSC.
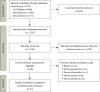
Methods: We conducted searches across three databases (PubMed, Embase, Cochrane) for randomized controlled studies up to June 23, 2024. A meta-analysis was performed on variables including adverse events, mortality, changes in the PaO2/FiO2 ratio, intensive care unit (ICU), length of stay, ventilation-free days, and changes in pro-inflammatory and anti-inflammatory cytokines. Relative risk (RR) values were employed for dichotomous variables, while mean difference (MD) and standard mean difference (SMD) were used for continuous variables. Risk bias was assessed using risk of bias 2 (ROB2).
Results: The meta-analysis encompassed 17 experiments involving 796 patients, with 410 undergoing MSC treatment and 386 in the control group. Primary outcomes indicated that MSC treatment did not escalate adverse events [RR =1.04; 95% confidence interval (CI): 0.90, 1.19; P=0.59; I2=0%]. On the contrary, it significantly diminishes the mortality (RR =0.79; 95% CI: 0.64, 0.97; P=0.02; I2=0%). Regarding secondary outcomes, MSCs led to a significant improvement in the PaO2/FiO2 ratio for ARDS patients (SMD =0.53; 95% CI: 0.15, 0.92; P=0.007; I2=0%). However, there were no significant differences in ICU length of stay (MD =-1.77; 95% CI: -6.97, 3.43; P=0.50; I2=63%) and ventilation-free days (MD =-1.29; 95% CI: -4.09, 1.51; P=0.37; I2=0%). MSCs significantly lowered C-reactive protein (CRP) (SMD =-0.65; 95% CI: -1.18, -0.13; P=0.01; I2=56%) and interleukin-6 (IL-6) levels compared to the control group (SMD =-0.76; 95% CI: -1.34, -0.17; P=0.01; I2=74%). However, changes in interleukin-10 (AIL-10) (SMD =-0.46; 95% CI: -1.51, 0.58; P=0.38; I2=77%), and changes in tumor necrosis factor-alpha (ATNF-α) (SMD =-1.5; 95% CI: -3.39, 0.40; P=0.12; I2=92%) levels showed no significant changes.
Conclusions: MSC therapy demonstrates reliable safety, with a significant impact on reducing mortality and improving certain clinical symptoms. Moreover, in certain aspects, it may alleviate the inflammatory response in ARDS. Nonetheless, these findings necessitate validation through additional high-quality randomized controlled trials.
Keywords: Acute respiratory distress syndrome (ARDS); adverse events; mesenchymal stem cells (MSCs); meta-analysis; mortality.
2024 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-281/coif). All authors report that this study was supported by National Key R&D Program of China (No. 2022YFC2504405), the Clinical Science and Technology Specific Projects of Jiangsu Province (BE2020786), the National Natural Science Foundation of China (Nos. 81870066 and 82270083), the Second Level Talents of the “333 High Level Talents Training Project” in the sixth phase in Jiangsu (LGY2022025), and Jiangsu Provincial Medical Key Laboratory (ZDXYS202205). The authors have no other conflicts of interest to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
