Recent research progresses of bioengineered biliary stents
- PMID: 39444940
- PMCID: PMC11497374
- DOI: 10.1016/j.mtbio.2024.101290
Recent research progresses of bioengineered biliary stents
Abstract
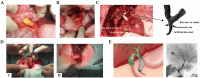
Bile duct lesion, including benign (eg. occlusion, cholelithiasis, dilatation, malformation) and malignant (cholangiocarcinoma) diseases, is a frequently encountered challenge in hepatobiliary diseases, which can be repaired by interventional or surgical procedures. A viable cure for bile duct lesions is implantation with biliary stents. Despite the placement achieved by current clinical biliary stents, the creation of functional and readily transplantable biliary stents remains a formidable obstacle. Excellent biocompatibility, stable mechanics, and absorbability are just a few benefits of using bioengineered biliary stents, which can also support and repair damaged bile ducts that drain bile. Additionally, cell sources & organoids derived from the biliary system that are loaded onto scaffolds can encourage bile duct regeneration. Therefore, the implantation of bioengineered biliary stent is considered as an ideal treatment for bile duct lesion, holding a broad potential for clinical applications in future. In this review, we look back on the development of conventional biliary stents, biodegradable biliary stents, and bioengineered biliary stents, highlighting the crucial elements of bioengineered biliary stents in promoting bile duct regeneration. After providing an overview of the various types of cell sources & organoids and fabrication methods utilized for the bioengineering process, we present the in vitro and in vivo applications of bioengineered biliary ducts, along with the latest advances in this exciting field. Finally, we also emphasize the ongoing challenges and future development of bioengineered biliary stents.
Keywords: 3D bio-printing; Biliary cell sources; Biodegradable stents; Bioengineering materials; Stent fabrication.
© 2024 The Authors.
Conflict of interest statement
All authors state no interest of this work.
Figures





References
-
- Hundt M., Basit H., John S. Treasure Island (FL) Ineligible Companies. Disclosure: Hajira Basit Declares No Relevant Financial Relationships with Ineligible Companies. Disclosure: Savio John Declares No Relevant Financial Relationships with Ineligible companies.: StatPearls Publishing Copyright © 2024. StatPearls Publishing LLC.; 2024. Physiology, bile secretion. StatPearls.
-
- Nakai Y., Kogure H., Yamada A., Isayama H., Koike K. Endoscopic management of bile duct stones in patients with surgically altered anatomy. Dig. Endosc. 2018;30(Suppl 1):67–74. - PubMed
-
- Eikermann M., Siegel R., Broeders I., Dziri C., Fingerhut A., Gutt C., Jaschinski T., Nassar A., Paganini A.M., Pieper D., et al. Prevention and treatment of bile duct injuries during laparoscopic cholecystectomy: the clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) Surg. Endosc. 2012;26(11):3003–3039. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

