Development of a luminescence-based method for measuring West Nile Virus MTase activity and its application to screen for antivirals
- PMID: 39445035
- PMCID: PMC11497361
- DOI: 10.1016/j.crmicr.2024.100282
Development of a luminescence-based method for measuring West Nile Virus MTase activity and its application to screen for antivirals
Abstract
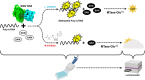
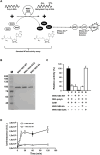
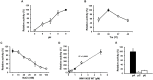
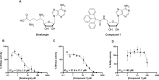
West Nile virus (WNV) is a flavivirus responsible for causing febrile illness and severe neurological diseases, with an increasing impact on human health around the world. However, there is still no adequate therapeutic treatment available to struggle WNV infections. Therefore, there is an urgent need to develop new techniques to accelerate the discovery of drugs against this pathogen. The main protein implicated in the replication of WNV is the non-structural protein 5 (NS5). This multifunctional protein contains methyltransferase (MTase) activity involved in the capping formation at the 5'-end of RNA and the methylation of internal viral RNA residues, both functions being essential for viral processes, such as RNA translation and escape from the innate immune response. We have developed a straightforward luminescence-based assay to monitor the MTase activity of the WNV NS5 protein with potential for high-throughput screening. We have validated this method as a sensitive and suitable assay for the identification of WNV MTase inhibitors assessing the inhibitory effect of the broad MTase inhibitor sinefungin, a natural nucleoside analog of the universal methyl donor S-adenosyl methionine (SAM). The screening of a small series of purine derivatives identified an adenosine derivative as a dose-dependent inhibitor of the MTase activity. The antiviral efficacy of this compound was further confirmed in WNV infections, displaying a measurable antiviral effect. This result supports the utility of this novel method for the screening of inhibitors against WNV MTase activity, which can be of special relevance to the discovery and development of therapeutics against WNV.
Keywords: NS5; WNV; antiviral; flavivirus; methyltransferase.
© 2024 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

