Survival outcomes of patients with diffuse large B-cell lymphoma undergoing autologous stem cell transplantation in Germany: real-world evidence from an administrative database between 2010 and 2019
- PMID: 39445061
- PMCID: PMC11496151
- DOI: 10.3389/fonc.2024.1432310
Survival outcomes of patients with diffuse large B-cell lymphoma undergoing autologous stem cell transplantation in Germany: real-world evidence from an administrative database between 2010 and 2019
Abstract
Background: Limited real-world evidence is available for patients with diffuse large B-cell lymphoma (DLBCL) who received an autologous stem cell transplantation (ASCT) in Germany.
Objectives: This study aims to describe the real-world survival outcomes of patients with DLBCL who received ASCT in Germany after diagnosis.
Design: This study is a retrospective database analysis covering the period between 2010 and 2019.
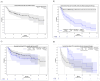
Methods: Unadjusted overall survival (OS) was plotted using the Kaplan-Meier estimator for the overall population and stratified by relapse status. A Cox regression was run to identify factors that influence OS.
Results: A total of 112 patients received an ASCT, with the average time from first-line treatment to ASCT being 11.7 months. The median OS estimated by Kaplan-Meier was 83.4 months for the entire cohort. The only variable that significantly reduced the OS was the presence of subsequent treatment after ASCT in a time-dependent model.
Conclusion: OS after ASCT for DLBCL patients in Germany is higher than previously reported and may still be considered a valid option for carefully selected patients with relapsed/refractory DLBCL.
Keywords: Germany; autologous stem cell transplantation; claims data; diffuse large B-cell lymphoma; real-world evidence; survival.
Copyright © 2024 Heger, Borchmann, Riou, Werner, Papadimitrious and Mahlich.
Conflict of interest statement
JM, SR, and MP are employees of Miltenyi Biomedicine GmbH. BW is an employee of Team Gesundheit, a contract research company that received funding from Miltenyi Biomedicine to conduct the study in line with the study protocol. The authors declare that this study received funding from Miltenyi Biomedicine. The funder had the following involvement in the study: three authors received salaries from Miltenyi Biomedicine. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Kamdar MK, Solomon S, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel (liso-cel) vs standard of care (SOC) with salvage chemotherapy (CT) followed by autologous stem cell transplantation (ASCT) as second-line (2L) treatment in patients (pt) with R/R large B-cell lymphoma (LBCL): 3-year follow-up (FU) from the randomized, phase 3 TRANSFORM study. JCO. (2024) 42:7013–3. doi: 10.1200/JCO.2024.42.16_suppl.7013 - DOI
-
- Tun A, Maliske S, Wang Y, Inwards DJ, Habermann TM, Micallef I, et al. Progression-free survival at 24 months as A landmark after autologous stem cell transplant in relapsed or refractory diffuse large B-cell lymphoma. Transplant Cell Ther. (2022) 28:610–7. doi: 10.1016/j.jtct.2022.06.015 - DOI - PubMed
-
- Wullenkord R, Berning P, Niemann AL, Wethmar K, Bergmann S, Lutz M, et al. The role of autologous stem cell transplantation (ASCT) in aggressive B-cell lymphomas: real-world data from a retrospective single-center analysis. Ann Hematol. (2021) 100:2733–44. doi: 10.1007/s00277-021-04650-5 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

