The use of 7T MRI in multiple sclerosis: review and consensus statement from the North American Imaging in Multiple Sclerosis Cooperative
- PMID: 39445084
- PMCID: PMC11497623
- DOI: 10.1093/braincomms/fcae359
The use of 7T MRI in multiple sclerosis: review and consensus statement from the North American Imaging in Multiple Sclerosis Cooperative
Abstract
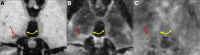
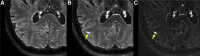
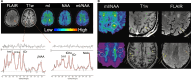
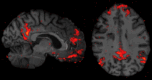
The use of ultra-high-field 7-Tesla (7T) MRI in multiple sclerosis (MS) research has grown significantly over the past two decades. With recent regulatory approvals of 7T scanners for clinical use in 2017 and 2020, the use of this technology for routine care is poised to continue to increase in the coming years. In this context, the North American Imaging in MS Cooperative (NAIMS) convened a workshop in February 2023 to review the previous and current use of 7T technology for MS research and potential future research and clinical applications. In this workshop, experts were tasked with reviewing the current literature and proposing a series of consensus statements, which were reviewed and approved by the NAIMS. In this review and consensus paper, we provide background on the use of 7T MRI in MS research, highlighting this technology's promise for identification and quantification of aspects of MS pathology that are more difficult to visualize with lower-field MRI, such as grey matter lesions, paramagnetic rim lesions, leptomeningeal enhancement and the central vein sign. We also review the promise of 7T MRI to study metabolic and functional changes to the brain in MS. The NAIMS provides a series of consensus statements regarding what is currently known about the use of 7T MRI in MS, and additional statements intended to provide guidance as to what work is necessary going forward to accelerate 7T MRI research in MS and translate this technology for use in clinical practice and clinical trials. This includes guidance on technical development, proposals for a universal acquisition protocol and suggestions for research geared towards assessing the utility of 7T MRI to improve MS diagnostics, prognostics and therapeutic efficacy monitoring. The NAIMS expects that this article will provide a roadmap for future use of 7T MRI in MS.
Keywords: 7 Tesla; magnetic resonance imaging; multiple sclerosis; ultra-high field.
Published by Oxford University Press on behalf of the Guarantors of Brain 2024.
Conflict of interest statement
D.M.H. has received research funding from EMD-Serono and Roche-Genentech, consulting fees from Horizon Therapeutics, TG Therapeutics and EMD-Serono and royalties from Up To Date, Inc. F.B. has received speaker honoraria from EMD-Serono, Sanofi and Novartis and serves/ed as site PI of multi-centre studies sponsored by EMD-Serono and Novartis and on advisory boards for Sanofi, EMD-Serono and Biogen. S.N. has received research funding from Roche-Genentech and Immunotec, consulting fees from Sana Biotechnology and personal compensation from NeuroRx Research. S.G. has received research funding from Roche-Genentech. E.S.B. has received consulting fees from EMD-Serono. .J.Z. has received research support from Novartis, I-Mab Biopharma and the Race to Erase MS Foundation. R.B. has received speaking honoraria from EMD-Serono and research support from Bristol-Myers Squibb, EMD-Serono and Novartis. A.C. was supported by the ECTRIMS post-doctoral training fellowship (2022). A.C. has received speaker honoraria from Novartis. S.Y.H. has received research funding and consulting fees from Siemens Healthineers. The University Hospital Basel (USB), as the employer of C.G., has received the following fees which were used exclusively for research support: (i) advisory board and consultancy fees from Actelion, Genzyme-Sanofi, Novartis, GeNeuro and Roche; (ii) speaker fees from Genzyme-Sanofi, Novartis, GeNeuro and Roche; and (iii) research support from Siemens, GeNeuro and Roche. E.C.K. has received research funding from Abbvie, Biogen and Genentech and consulting fees from EMD-Serono, Genentech, INmune Bio, Myrobalan Therapeutics, OM1, Inc. and TG Therapeutics. C.M. has received research funding from Genentech-Roche. C.L., J.D., M.D., D.A.R. and P.B. have no disclosures to report.
Figures




References
-
- Li TQ, van Gelderen P, Merkle H, Talagala L, Koretsky AP, Duyn J. Extensive heterogeneity in white matter intensity in high-resolution T2*-weighted MRI of the human brain at 7.0 T. Neuroimage. 2006;32:1032–1040. - PubMed
-
- Hammond KE, Metcalf M, Carvajal L, et al. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann Neurol. 2008;64:707–713. - PubMed
-
- Tallantyre EC, Brookes MJ, Dixon JE, Morgan PS, Evangelou N, Morris PG. Demonstrating the perivascular distribution of MS lesions in vivo with 7-Tesla MRI. Neurology. 2008;70:2076–2078. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
