Patient, Care Partner, and Physician Voices in Treatment Decision-Making for Multiple Myeloma
- PMID: 39445100
- PMCID: PMC11498144
- DOI: 10.2147/PPA.S474722
Patient, Care Partner, and Physician Voices in Treatment Decision-Making for Multiple Myeloma
Abstract
Introduction: Treatment decision-making for multiple myeloma (MM) is complex. Individuals involved in decision-making may value treatment attributes differently based on their role as a patient, care partner, or physician. This study describes those attributes, and what is most important by role.
Methods: We conducted a cross-sectional online survey with consenting adult patients with MM, MM care partners, and physicians treating MM. Respondents were recruited from US panels (Inspire and M3 Global Research) between September and December 2022. Survey items were informed by a targeted literature review, qualitative interviews, and a steering committee comprising clinical experts, a patient advocate, patient, and care partner. Descriptive statistics were generated and reported in aggregate.
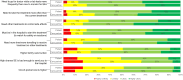
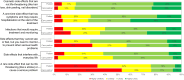
Results: Email invitations were sent to 8071 Inspire members interested in or posting about MM. Of these, 4427 viewed the invitation, 941 responded, and 156 patients and care partners completed the survey (17% of respondents). For physicians, 5588 were invited via Email by M3 Global Research, with 761 viewing the invitation, 214 accessing the survey link, and 137 completing the survey (64% of respondents). Duration of response, side effects, and patients' quality-of-life (QoL) were the top three treatment attributes selected across the three cohorts; alignment of these attributes was consistent among patients regardless of disease severity. Separately, patients rated QoL and the amount of caregiving needed during/after treatment as the most important factors for future treatment decisions. If more effective MM treatments were offered, care partners were more willing to assume greater family burden (77%) compared to patients (49%), and patients were more accepting of potential serious side effects (50%) than were care partners (34%).
Conclusion: Patients with MM, care partners, and physicians consider and value various treatment decision-making factors. Recognizing and addressing these differences is critical to meeting patients' preferences, needs, and optimizing patient outcomes.
Keywords: Decision-making; cross-sectional survey; multiple myeloma; treatment choice.
© 2024 Dwyer Orr et al.
Conflict of interest statement
LDO, DL, BW are current employees of Janssen Scientific Affairs, LLC. KLD, MY, and VK are employees of EPI-Q Inc., which received funding from Janssen Scientific Affairs, LLC associated with the development and execution of this study. JF and EK are current or former employees of Inspire Insights, which received funding associated with the development and execution of this study. TWL has received honoraria for consulting/advisory boards from AbbVie, Agilix, Agios/Servier, Apellis, Astellas, AstraZeneca, Beigene, BlueNote, BMS/Celgene, CareVive, Flatiron, Genentech, GSK, Lilly, Meter Health, Novartis, and Pfizer; speaking-related honoraria from AbbVie, Agios, Astellas, BMS/Celgene, and Incyte; equity interest in Dosentrx (stock options in a privately-held company); royalties from UpToDate; research funding from the AbbVie, American Cancer Society, AstraZeneca, BMS, Deverra Therapeutics, Duke University, GSK, Jazz Pharmaceuticals, Rigel, the Leukemia and Lymphoma Society, the National Institute of Nursing Research/ National Institutes of Health, and Seattle Genetics. JA has served on advisory boards for BMS, J&J, Pfizer, Regeneron, Sanofi, and Takeda Oncology. BF is a consultant to GSK, Janssen, Sanofi, and Pfizer. NB has served consulting/advisory roles for AbbVie, BMS, Janssen, Pfizer, and Sanofi; and, received research funding from Amgen, BMS, Karyopharm Therapeutics, and Merck. The authors report no other conflicts of interest in this work.
Figures
References
-
- Surveillance Epidemiology, and End Results (SEER). Cancer stat facts: myeloma. Available from: https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed August 8, 2023.
LinkOut - more resources
Full Text Sources