Engineered AAV capsid transport mutants overcome transduction deficiencies in the aged CNS
- PMID: 39445231
- PMCID: PMC11497394
- DOI: 10.1016/j.omtn.2024.102332
Engineered AAV capsid transport mutants overcome transduction deficiencies in the aged CNS
Abstract
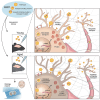
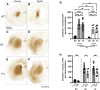
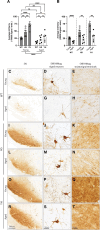
Adeno-associated virus (AAV)-based gene therapy has enjoyed great successes over the past decade, with Food and Drug Administration-approved therapeutics and a robust clinical pipeline. Nonetheless, barriers to successful translation remain. For example, advanced age is associated with impaired brain transduction, with the diminution of infectivity depending on anatomical region and capsid. Given that CNS gene transfer is often associated with neurodegenerative diseases where age is the chief risk factor, we sought to better understand the causes of this impediment. We assessed two AAV variants hypothesized to overcome factors negatively impacting transduction in the aged brain; specifically, changes in extracellular and cell-surface glycans, and intracellular transport. We evaluated a heparin sulfate proteoglycan null variant with or without mutations enhancing intracellular transport. Vectors were injected into the striatum of young adult or aged rats to address whether improving extracellular diffusion, removing glycan receptor dependence, or improving intracellular transport are important factors in transducing the aged brain. We found that, regardless of the viral capsid, there was a reduction in many of our metrics of transduction in the aged brain. However, the transport mutant was less sensitive to age, suggesting that changes in the cellular transport of AAV capsids are a key factor in age-related transduction deficiency.
Keywords: AAV; CNS; MT: Delivery Strategies; aging; capsid mutation; diffusion; receptor; retrograde transduction; transport.
© 2024 The Author(s).
Conflict of interest statement
I.M.S.: Co-founder of nVector. Has received financial support from Aspen Neurosciences. F.P.M.: Co-founder of nVector Therapeutics, CavGene Therapeutics, and Neuralina Therapeutics. Has received financial support from Regenex Bio, Aspen Neurosciences, Seelos Therapeutics. K.S.-C.: Co-founder of CavGene Therapeutics, Inc, which holds intellectual property in CaV1.3 gene silencing and has received financial support from Regenex Bio. S.E.B.: Co-founder of Atsena Therapeutics. S.L.B.: Co-founder of Atsena Therapeutics. M.D.: Co-founder of rAAVEN. D.J.M.: Has received financial support from FujiFilm Cellular Dynamics Inc and Aspen Neurosciences. Currently an employee of Biogen. I.M.S., F.P.M., S.E.B., S.L.B., and M.D. hold patents related to AAV technology.
Figures




References
-
- Al-Zaidy S., Pickard A.S., Kotha K., Alfano L.N., Lowes L., Paul G., Church K., Lehman K., Sproule D.M., Dabbous O., et al. Health outcomes in spinal muscular atrophy type 1 following AVXS-101 gene replacement therapy. Pediatr. Pulmonol. 2019;54:179–185. doi: 10.1002/ppul.24203. - DOI - PMC - PubMed
-
- Bartus R.T., Herzog C.D., Chu Y., Wilson A., Brown L., Siffert J., Johnson E.M., Jr., Olanow C.W., Mufson E.J., Kordower J.H. Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson's disease and nonhuman primate brains. Mov. Disord. 2011;26:27–36. doi: 10.1002/mds.23442. - DOI - PMC - PubMed
-
- Marks W.J., Jr., Bartus R.T., Siffert J., Davis C.S., Lozano A., Boulis N., Vitek J., Stacy M., Turner D., Verhagen L., et al. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

