Associations of semaglutide with first-time diagnosis of Alzheimer's disease in patients with type 2 diabetes: Target trial emulation using nationwide real-world data in the US
- PMID: 39445596
- PMCID: PMC11667504
- DOI: 10.1002/alz.14313
Associations of semaglutide with first-time diagnosis of Alzheimer's disease in patients with type 2 diabetes: Target trial emulation using nationwide real-world data in the US
Abstract
Introduction: Emerging preclinical evidence suggests that semaglutide, a glucagon-like peptide receptor agonist (GLP-1RA) for type 2 diabetes mellitus (T2DM) and obesity, protects against neurodegeneration and neuroinflammation. However, real-world evidence for its ability to protect against Alzheimer's disease (AD) is lacking.
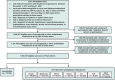
Methods: We conducted emulation target trials based on a nationwide database of electronic health records (EHRs) of 116 million US patients. Seven target trials were emulated among 1,094,761 eligible patients with T2DM who had no prior AD diagnosis by comparing semaglutide with seven other antidiabetic medications. First-ever diagnosis of AD occurred within a 3-year follow-up period and was examined using Cox proportional hazards and Kaplan-Meier survival analyses.
Results: Semaglutide was associated with significantly reduced risk for first-time AD diagnosis, most strongly compared with insulin (hazard ratio [HR], 0.33 [95% CI: 0.21 to 0.51]) and most weakly compared with other GLP-1RAs (HR, 0.59 [95% CI: 0.37 to 0.95]). Similar results were seen across obesity status, gender, and age groups.
Discussion: These findings support further studies to assess semaglutide's potential in preventing AD.
Highlights: Semaglutide was associated with 40% to 70% reduced risks of first-time AD diagnosis in T2DM patients compared to other antidiabetic medications, including other GLP-1RAs. Semaglutide was associated with significantly lower AD-related medication prescriptions. Similar reductions were seen across obesity status, gender, and age groups. Our findings provide real-world evidence supporting the potential clinical benefits of semaglutide in mitigating AD initiation and development in patients with T2DM. These findings support further clinical trials to assess semaglutide's potential in delaying or preventing AD.
Keywords: Alzheimer's disease; emulation target trial; patient electronic health records; prevention; real‐world data; semaglutide; type 2 diabetes.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
All authors declare no competing interests. Author disclosures are available in the Supporting Information.
Figures



References
-
- Committee on the Public Health Dimensions of Cognitive Aging , Board on Health Sciences Policy , Institute of Medicine . Cognitive Aging: Progress in Understanding and Opportunities for Action. In Blazer DG, Yaffe K, Liverman CT, eds. National Academies Press (US); 2015. - PubMed
-
- World Health Organization . Risk reduction of cognitive decline and dementia: WHO guidelines. World Health Organization. Published January 1, 2019. Accessed June 29, 2024. https://www.who.int/publications/i/item/9789241550543 - PubMed
-
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

